Lab Hra Pharma Company Profile
✉ Email this page to a colleague
What is the competitive landscape for LAB HRA PHARMA, and what generic alternatives to LAB HRA PHARMA drugs are available?
LAB HRA PHARMA has one approved drug.
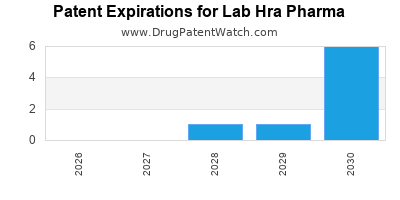
There are eight US patents protecting LAB HRA PHARMA drugs.
There are fifty-five patent family members on LAB HRA PHARMA drugs in twenty-eight countries.
Drugs and US Patents for Lab Hra Pharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lab Hra Pharma | ELLA | ulipristal acetate | TABLET;ORAL | 022474-001 | Aug 13, 2010 | AB | RX | Yes | Yes | 10,772,897 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Lab Hra Pharma | ELLA | ulipristal acetate | TABLET;ORAL | 022474-001 | Aug 13, 2010 | AB | RX | Yes | Yes | 8,426,392 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Lab Hra Pharma | ELLA | ulipristal acetate | TABLET;ORAL | 022474-001 | Aug 13, 2010 | AB | RX | Yes | Yes | 9,283,233 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Lab Hra Pharma | ELLA | ulipristal acetate | TABLET;ORAL | 022474-001 | Aug 13, 2010 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Lab Hra Pharma | ELLA | ulipristal acetate | TABLET;ORAL | 022474-001 | Aug 13, 2010 | AB | RX | Yes | Yes | 10,159,681 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Lab Hra Pharma | ELLA | ulipristal acetate | TABLET;ORAL | 022474-001 | Aug 13, 2010 | AB | RX | Yes | Yes | 9,844,510 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Lab Hra Pharma | ELLA | ulipristal acetate | TABLET;ORAL | 022474-001 | Aug 13, 2010 | AB | RX | Yes | Yes | 8,735,380 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Paragraph IV (Patent) Challenges for LAB HRA PHARMA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 30 mg | ➤ Subscribe | 2014-08-13 |
International Patents for Lab Hra Pharma Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 3106167 | ⤷ Try a Trial |
| Mexico | 343358 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2010119029 | ⤷ Try a Trial |
| Poland | 2419108 | ⤷ Try a Trial |
| Slovenia | 2365800 | ⤷ Try a Trial |
| Colombia | 6400186 | ⤷ Try a Trial |
| South Africa | 201107543 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Similar Applicant Names
Here is a list of applicants with similar names.