Janssen Biotech Company Profile
✉ Email this page to a colleague
What is the competitive landscape for JANSSEN BIOTECH, and when can generic versions of JANSSEN BIOTECH drugs launch?
JANSSEN BIOTECH has four approved drugs.
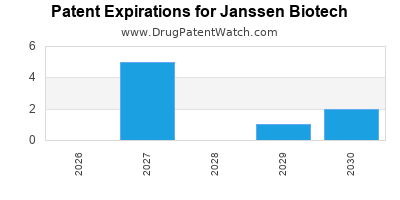
There are twenty-four US patents protecting JANSSEN BIOTECH drugs.
There are six hundred and forty-one patent family members on JANSSEN BIOTECH drugs in fifty-nine countries and seventy-three supplementary protection certificates in twenty countries.
Summary for Janssen Biotech
| International Patents: | 641 |
| US Patents: | 24 |
| Tradenames: | 4 |
| Ingredients: | 4 |
| NDAs: | 4 |
| Patent Litigation for Janssen Biotech: | See patent lawsuits for Janssen Biotech |
Drugs and US Patents for Janssen Biotech
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Biotech | BALVERSA | erdafitinib | TABLET;ORAL | 212018-002 | Apr 12, 2019 | RX | Yes | No | 11,684,620 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen Biotech | AKEEGA | abiraterone acetate; niraparib tosylate | TABLET;ORAL | 216793-002 | Aug 11, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Janssen Biotech | ERLEADA | apalutamide | TABLET;ORAL | 210951-002 | Feb 17, 2023 | RX | Yes | Yes | 10,702,508 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Janssen Biotech
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Biotech | ZYTIGA | abiraterone acetate | TABLET;ORAL | 202379-001 | Apr 28, 2011 | 5,604,213 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for JANSSEN BIOTECH drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 250 mg | ➤ Subscribe | 2015-04-28 |
| ➤ Subscribe | Tablets | 500 mg | ➤ Subscribe | 2017-08-23 |
International Patents for Janssen Biotech Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Jordan | P20190190 | ⤷ Try a Trial |
| Spain | 2588606 | ⤷ Try a Trial |
| South Korea | 20200014736 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Janssen Biotech Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2368550 | 1990032-3 | Sweden | ⤷ Try a Trial | PRODUCT NAME: APALUTAMIDE; REG. NO/DATE: EU/1/1342 20190116 |
| 2109608 | LUC00072 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: NIRAPARIB OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE, STEREOISOMERE OU TAUTOMERE DE CELUI-CI, NOTAMMENT LE TOSYLATE OU UN HYDRATE, PARTICULIEREMENT LE TOSYLATE MONOHYDRATE; AUTHORISATION NUMBER AND DATE: EU/1/17/1235 20171120 |
| 2109608 | CA 2018 00017 | Denmark | ⤷ Try a Trial | PRODUCT NAME: NIRAPARIB OR A PHARMACEUTICALLY ACCEPTABLE SALT, STEREOISOMER OR TAUTOMER THEREOF, PARTICULARLY THE; REG. NO/DATE: EU/1/17/1235 20171120 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.