Intra-cellular Company Profile
✉ Email this page to a colleague
What is the competitive landscape for INTRA-CELLULAR, and when can generic versions of INTRA-CELLULAR drugs launch?
INTRA-CELLULAR has one approved drug.
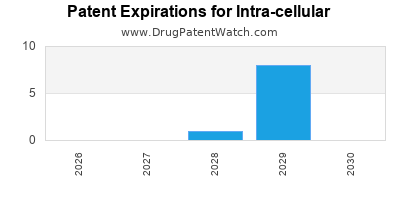
There are eighteen US patents protecting INTRA-CELLULAR drugs.
There are one hundred and thirty-eight patent family members on INTRA-CELLULAR drugs in twenty countries.
Summary for Intra-cellular
| International Patents: | 138 |
| US Patents: | 18 |
| Tradenames: | 1 |
| Ingredients: | 1 |
| NDAs: | 1 |
Drugs and US Patents for Intra-cellular
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-cellular | CAPLYTA | lumateperone tosylate | CAPSULE;ORAL | 209500-003 | Apr 22, 2022 | RX | Yes | No | 11,052,084 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Intra-cellular | CAPLYTA | lumateperone tosylate | CAPSULE;ORAL | 209500-003 | Apr 22, 2022 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Intra-cellular | CAPLYTA | lumateperone tosylate | CAPSULE;ORAL | 209500-002 | Apr 22, 2022 | RX | Yes | No | 9,168,258 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Intra-cellular | CAPLYTA | lumateperone tosylate | CAPSULE;ORAL | 209500-001 | Dec 20, 2019 | RX | Yes | Yes | 11,753,419 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Intra-cellular | CAPLYTA | lumateperone tosylate | CAPSULE;ORAL | 209500-001 | Dec 20, 2019 | RX | Yes | Yes | 11,806,348 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Intra-cellular | CAPLYTA | lumateperone tosylate | CAPSULE;ORAL | 209500-002 | Apr 22, 2022 | RX | Yes | No | RE48825 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Intra-cellular | CAPLYTA | lumateperone tosylate | CAPSULE;ORAL | 209500-002 | Apr 22, 2022 | RX | Yes | No | 9,199,995 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Intra-cellular
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Intra-cellular | CAPLYTA | lumateperone tosylate | CAPSULE;ORAL | 209500-001 | Dec 20, 2019 | 7,183,282 | ⤷ Try a Trial |
| Intra-cellular | CAPLYTA | lumateperone tosylate | CAPSULE;ORAL | 209500-001 | Dec 20, 2019 | RE39680 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Intra-cellular Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Poland | 2262505 | ⤷ Try a Trial |
| Mexico | 2009009773 | ⤷ Try a Trial |
| Israel | 246002 | ⤷ Try a Trial |
| Israel | 305990 | ⤷ Try a Trial |
| Russian Federation | 2682658 | ⤷ Try a Trial |
| South Korea | 101999300 | ⤷ Try a Trial |
| Croatia | P20141178 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Similar Applicant Names
Here is a list of applicants with similar names.