Incyte Corp Company Profile
✉ Email this page to a colleague
What is the competitive landscape for INCYTE CORP, and what generic alternatives to INCYTE CORP drugs are available?
INCYTE CORP has three approved drugs.
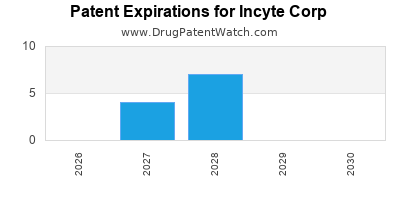
There are twenty-four US patents protecting INCYTE CORP drugs.
There are three hundred and ninety-four patent family members on INCYTE CORP drugs in fifty countries and forty-one supplementary protection certificates in eighteen countries.
Drugs and US Patents for Incyte Corp
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-001 | Nov 16, 2011 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Incyte Corp | PEMAZYRE | pemigatinib | TABLET;ORAL | 213736-001 | Apr 17, 2020 | RX | Yes | No | 11,628,162 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-004 | Nov 16, 2011 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Incyte Corp | PEMAZYRE | pemigatinib | TABLET;ORAL | 213736-002 | Apr 17, 2020 | RX | Yes | No | 9,611,267 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Paragraph IV (Patent) Challenges for INCYTE CORP drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 5 mg, 10 mg, 15 mg, 20 mg, and 25 mg | ➤ Subscribe | 2015-12-17 |
International Patents for Incyte Corp Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Taiwan | I630207 | ⤷ Try a Trial |
| European Patent Office | 3184526 | ⤷ Try a Trial |
| European Patent Office | 2740731 | ⤷ Try a Trial |
| Eurasian Patent Organization | 035981 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Incyte Corp Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2861595 | C02861595/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: PEMIGATINIB; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 68143 13.07.2021 |
| 1966202 | C 2013 005 | Romania | ⤷ Try a Trial | PRODUCT NAME: RUXOLITINIB SAU O SARE FARMACEUTIC ACCEPTABILA AACESTUIA; NATIONAL AUTHORISATION NUMBER: RO EU/1/12/773/001, RO EU/1/12/773/002, RO EU/1/12/773/003; DATE OF NATIONAL AUTHORISATION: 20120823; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EMEA EU/1/12/773/001, EMEA EU/1/12/773/002, EMEA EU/1/12/773/003; DATE OF FIRST AUTHORISATION IN EEA: 20120823 |
| 2861595 | CR 2021 00033 | Denmark | ⤷ Try a Trial | PRODUCT NAME: PEMIGATINIB ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/21/1535 20210329 |
| 2455382 | PA2017012 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: RUKSOLITINIBAS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/12/773/001-006 20150311 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.