Hoffmann La Roche Company Profile
✉ Email this page to a colleague
What is the competitive landscape for HOFFMANN LA ROCHE, and when can generic versions of HOFFMANN LA ROCHE drugs launch?
HOFFMANN LA ROCHE has ten approved drugs.
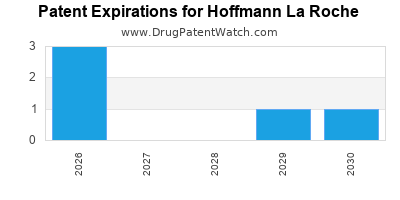
There are six US patents protecting HOFFMANN LA ROCHE drugs.
There are one hundred and eighty patent family members on HOFFMANN LA ROCHE drugs in forty-six countries and twenty-three supplementary protection certificates in seventeen countries.
Summary for Hoffmann La Roche
| International Patents: | 180 |
| US Patents: | 6 |
| Tradenames: | 9 |
| Ingredients: | 8 |
| NDAs: | 10 |
Drugs and US Patents for Hoffmann La Roche
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hoffmann La Roche | ROCEPHIN | ceftriaxone sodium | INJECTABLE;INJECTION | 062654-003 | Apr 30, 1987 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Hoffmann La Roche | BONIVA | ibandronate sodium | TABLET;ORAL | 021455-002 | Mar 24, 2005 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Hoffmann La Roche | ACCUTANE | isotretinoin | CAPSULE;ORAL | 018662-002 | May 7, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Hoffmann La Roche | ROCEPHIN | ceftriaxone sodium | INJECTABLE;INTRAMUSCULAR, INTRAVENOUS | 050585-004 | Dec 21, 1984 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Hoffmann La Roche | ROCEPHIN W/ DEXTROSE IN PLASTIC CONTAINER | ceftriaxone sodium | INJECTABLE;INJECTION | 050624-001 | Feb 11, 1987 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Hoffmann La Roche
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Hoffmann La Roche | ACCUTANE | isotretinoin | CAPSULE;ORAL | 018662-002 | May 7, 1982 | 4,464,394*PED | ⤷ Try a Trial |
| Hoffmann La Roche | ACCUTANE | isotretinoin | CAPSULE;ORAL | 018662-004 | Mar 28, 1983 | 4,200,647 | ⤷ Try a Trial |
| Hoffmann La Roche | BONIVA | ibandronate sodium | TABLET;ORAL | 021455-001 | May 16, 2003 | 6,294,196 | ⤷ Try a Trial |
| Hoffmann La Roche | ACCUTANE | isotretinoin | CAPSULE;ORAL | 018662-004 | Mar 28, 1983 | 4,322,438 | ⤷ Try a Trial |
| Hoffmann La Roche | BONIVA | ibandronate sodium | TABLET;ORAL | 021455-002 | Mar 24, 2005 | 4,927,814 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for HOFFMANN LA ROCHE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 2.5 mg and 150 mg | ➤ Subscribe | 2007-05-16 |
| ➤ Subscribe | Injection | 1 mg/mL, 3 mL Vial | ➤ Subscribe | 2007-08-31 |
International Patents for Hoffmann La Roche Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 2778693 | ⤷ Try a Trial |
| South Africa | 201202937 | ⤷ Try a Trial |
| Denmark | 1696920 | ⤷ Try a Trial |
| China | 102603581 | ⤷ Try a Trial |
| Mexico | 2011008303 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Hoffmann La Roche Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1893612 | CA 2012 00028 | Denmark | ⤷ Try a Trial | |
| 1893612 | C300534 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: VEMURAFENIB ALSMEDE FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN; REGISTRATION NO/DATE: EU/1/12/751/001 20120217 |
| 1893612 | 28/2012 | Austria | ⤷ Try a Trial | PRODUCT NAME: VEMURAFENIB UND PHARMAZEUTISCH VERTRAEGLICHE SALZE DAVON; REGISTRATION NO/DATE: EU/1/12/751/001 (MITTEILUNG) 20120221 |
| 1893612 | C01893612/01 | Switzerland | ⤷ Try a Trial | VERTRETERLOESCHUNG |
| 1893612 | 12C0040 | France | ⤷ Try a Trial | PRODUCT NAME: VEMURAFENIB, EVENTUELLEMENT SOUS LA FORME D'UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/12/751/001 20120221 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.