HORIZON Company Profile
✉ Email this page to a colleague
What is the competitive landscape for HORIZON, and when can generic versions of HORIZON drugs launch?
HORIZON has nine approved drugs.
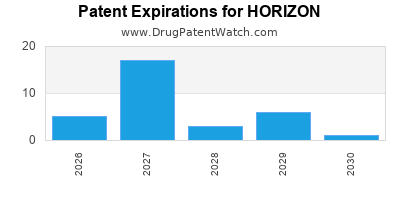
There are fifty-seven US patents protecting HORIZON drugs.
There are two hundred and thirty patent family members on HORIZON drugs in forty-four countries and one hundred and one supplementary protection certificates in eighteen countries.
Summary for HORIZON
| International Patents: | 230 |
| US Patents: | 57 |
| Tradenames: | 7 |
| Ingredients: | 7 |
| NDAs: | 9 |
| Patent Litigation for HORIZON: | See patent lawsuits for HORIZON |
| PTAB Cases with HORIZON as patent owner: | See PTAB cases with HORIZON as patent owner |
Drugs and US Patents for HORIZON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Horizon | PROCYSBI | cysteamine bitartrate | GRANULE, DELAYED RELEASE;ORAL | 213491-002 | Feb 14, 2020 | RX | Yes | Yes | 9,173,851*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Horizon | PROCYSBI | cysteamine bitartrate | CAPSULE, DELAYED RELEASE;ORAL | 203389-002 | Apr 30, 2013 | RX | Yes | Yes | 9,173,851*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Horizon | PROCYSBI | cysteamine bitartrate | CAPSULE, DELAYED RELEASE;ORAL | 203389-002 | Apr 30, 2013 | RX | Yes | Yes | 9,233,077*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-003 | Jul 26, 2012 | AB | RX | Yes | Yes | 8,309,124 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for HORIZON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Horizon | VIMOVO | esomeprazole magnesium; naproxen | TABLET, DELAYED RELEASE;ORAL | 022511-001 | Apr 30, 2010 | 7,745,466 | ⤷ Try a Trial |
| Horizon | VIMOVO | esomeprazole magnesium; naproxen | TABLET, DELAYED RELEASE;ORAL | 022511-002 | Apr 30, 2010 | 8,858,996 | ⤷ Try a Trial |
| Horizon | VIMOVO | esomeprazole magnesium; naproxen | TABLET, DELAYED RELEASE;ORAL | 022511-002 | Apr 30, 2010 | 7,411,070*PED | ⤷ Try a Trial |
| Horizon | RAYOS | prednisone | TABLET, DELAYED RELEASE;ORAL | 202020-001 | Jul 26, 2012 | 8,309,124 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for HORIZON drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Topical Solution | 1.5% | ➤ Subscribe | 2012-07-11 |
| ➤ Subscribe | Delayed-release Tablets | 1 mg, 2 mg, and 5 mg | ➤ Subscribe | 2012-11-26 |
| ➤ Subscribe | Tablets | 800 mg/26.6 mg | ➤ Subscribe | 2011-12-06 |
| ➤ Subscribe | Topical Solution | 2.0% | ➤ Subscribe | 2014-06-03 |
| ➤ Subscribe | Delayed-release Tablet | 375 mg/20 mg and 500 mg/20 mg | ➤ Subscribe | 2010-11-05 |
| ➤ Subscribe | Oral Liquid | 1.1 g/mL | ➤ Subscribe | 2013-11-19 |
International Patents for HORIZON Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Argentina | 096628 | ⤷ Try a Trial |
| Cyprus | 1113690 | ⤷ Try a Trial |
| European Patent Office | 1631251 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2010025303 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for HORIZON Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1919458 | 122014000023 | Germany | ⤷ Try a Trial | PRODUCT NAME: CYSTEAMIN ODER EIN PHARMAZEUTISCH VERTRAEGLICHES SALZ DAVON; REGISTRATION NO/DATE: EU/1/13/861 20130906 |
| 1499331 | 13C0055 | France | ⤷ Try a Trial | PRODUCT NAME: SULFATE DE SODIUM ANHYDRE, SULFATE DE MAGNESIUM HEPTAHYDRATE, SULFATE DE POTASSIUM; NAT. REGISTRATION NO/DATE: NL41696 20130426; FIRST REGISTRATION: BE - 434323 20130220 |
| 1411900 | SPC/GB11/015 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: NAPROXEN AND ESOMEPRAZOLE; REGISTERED: UK PL 17901/0263-0001 20101105 |
| 2340828 | LUC00195 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SACUBITRIL ET VALSARTAN, SOUS FORME DE COMPLEXE DE SEL DE SODIUM SACUBITRIL VALSARTAN, C'EST-A-DIRE (((S)-N-VALERYL-N-((2'-(1H-TETRAZOLE-5-YL)-BIPHENYL-4-YL)-METHYL)-VALINE) ((2R,4S)-5-BIPHENYL-4-YL-4-(3-CARBOXY-PROPIONYLAMINO)-2-METHYL-ESTER ETHYLIQUE D'ACIDE PENTANOIQUE))NA3 X H2O, DANS LEQUEL X EST 0 A 3; AUTHORISATION NUMBER AND DATE: EU/1/15/1058 20151123 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.