HELSINN HLTHCARE Company Profile
✉ Email this page to a colleague
What is the competitive landscape for HELSINN HLTHCARE, and what generic alternatives to HELSINN HLTHCARE drugs are available?
HELSINN HLTHCARE has five approved drugs.
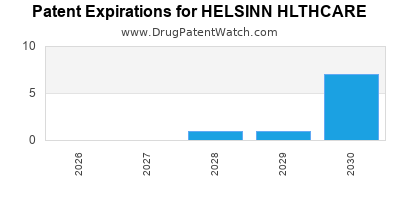
There are thirty-seven US patents protecting HELSINN HLTHCARE drugs.
There are three hundred and thirty-six patent family members on HELSINN HLTHCARE drugs in fifty-eight countries and twenty-three supplementary protection certificates in fourteen countries.
Summary for HELSINN HLTHCARE
| International Patents: | 336 |
| US Patents: | 37 |
| Tradenames: | 3 |
| Ingredients: | 4 |
| NDAs: | 5 |
Drugs and US Patents for HELSINN HLTHCARE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Helsinn Hlthcare | AKYNZEO | netupitant; palonosetron hydrochloride | CAPSULE;ORAL | 205718-001 | Oct 10, 2014 | RX | Yes | Yes | 8,951,969 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Helsinn Hlthcare | ALOXI | palonosetron hydrochloride | INJECTABLE;INTRAVENOUS | 021372-001 | Jul 25, 2003 | DISCN | Yes | No | 8,598,219*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Helsinn Hlthcare | AKYNZEO | fosnetupitant chloride hydrochloride; palonosetron hydrochloride | SOLUTION;INTRAVENOUS | 210493-002 | May 27, 2020 | RX | Yes | Yes | 10,624,911 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Helsinn Hlthcare | ALOXI | palonosetron hydrochloride | INJECTABLE;INTRAVENOUS | 021372-001 | Jul 25, 2003 | DISCN | Yes | No | 9,125,905*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for HELSINN HLTHCARE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Helsinn Hlthcare | AKYNZEO | netupitant; palonosetron hydrochloride | CAPSULE;ORAL | 205718-001 | Oct 10, 2014 | 5,202,333 | ⤷ Try a Trial |
| Helsinn Hlthcare | ALOXI | palonosetron hydrochloride | INJECTABLE;INTRAVENOUS | 021372-002 | Feb 29, 2008 | 9,125,905 | ⤷ Try a Trial |
| Helsinn Hlthcare | ALOXI | palonosetron hydrochloride | INJECTABLE;INTRAVENOUS | 021372-002 | Feb 29, 2008 | 5,202,333*PED | ⤷ Try a Trial |
| Helsinn Hlthcare | ALOXI | palonosetron hydrochloride | CAPSULE;ORAL | 022233-001 | Aug 22, 2008 | 5,202,333 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for HELSINN HLTHCARE Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Singapore | 153875 | ⤷ Try a Trial |
| Ukraine | 90449 | ⤷ Try a Trial |
| Japan | 2021183639 | ⤷ Try a Trial |
| Jordan | 3202 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for HELSINN HLTHCARE Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1035115 | C01035115/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: NETUPITANT; REGISTRATION NO/DATE: SWISSMEDIC 65499 06.11.2015 |
| 0430190 | SPC/GB05/032 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: PALONOSETRON OR AN ISOMER, MIXTURE OF ISOMERS, N-OXIDE OR PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/04/306/001 20050322 |
| 2785706 | SPC/GB20/037 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: FOSNETUPITANT; REGISTERED: UK EU/1/15/1001 (NI) 20200318; UK PLGB 12333/0017-0001 20200318 |
| 2785706 | 132020000000094 | Italy | ⤷ Try a Trial | PRODUCT NAME: FOSNETUPITANT O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE, IN PARTICOLARE IL SUO SALE CLORURO CLORIDRATO(AKYNZEO); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/15/1001/001-002, 20200316 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.