Glaxosmithkline Company Profile
✉ Email this page to a colleague
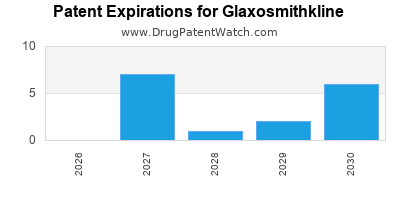
What is the competitive landscape for GLAXOSMITHKLINE, and when can generic versions of GLAXOSMITHKLINE drugs launch?
GLAXOSMITHKLINE has one hundred and forty-nine approved drugs.
There are thirty-two US patents protecting GLAXOSMITHKLINE drugs.
There are seven hundred and twenty-four patent family members on GLAXOSMITHKLINE drugs in fifty-nine countries and one hundred and eighty-five supplementary protection certificates in twenty countries.
Summary for Glaxosmithkline
| International Patents: | 724 |
| US Patents: | 32 |
| Tradenames: | 104 |
| Ingredients: | 90 |
| NDAs: | 149 |
| Drug Master File Entries: | 8 |
| Patent Litigation for Glaxosmithkline: | See patent lawsuits for Glaxosmithkline |
| PTAB Cases with Glaxosmithkline as petitioner: | See PTAB cases with Glaxosmithkline as petitioner |
Drugs and US Patents for Glaxosmithkline
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glaxosmithkline | TOTACILLIN-N | ampicillin sodium | INJECTABLE;INJECTION | 062727-001 | Dec 19, 1986 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Glaxosmithkline | BEEPEN-VK | penicillin v potassium | TABLET;ORAL | 062273-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Glaxosmithkline | ZEJULA | niraparib tosylate | TABLET;ORAL | 214876-001 | Apr 26, 2023 | RX | Yes | No | 8,143,241 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Glaxosmithkline | ZEJULA | niraparib tosylate | CAPSULE;ORAL | 208447-001 | Mar 27, 2017 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Glaxosmithkline | COMPAZINE | prochlorperazine edisylate | CONCENTRATE;ORAL | 011276-001 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Glaxosmithkline | DARBID | isopropamide iodide | TABLET;ORAL | 010744-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Glaxosmithkline
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Glaxosmithkline | SEREVENT | salmeterol xinafoate | POWDER;INHALATION | 020692-001 | Sep 19, 1997 | 5,860,419*PED | ⤷ Try a Trial |
| Glaxosmithkline | WELLBUTRIN SR | bupropion hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 020358-002 | Oct 4, 1996 | 5,358,970 | ⤷ Try a Trial |
| Glaxosmithkline | WELLBUTRIN SR | bupropion hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 020358-001 | Oct 4, 1996 | 5,763,493 | ⤷ Try a Trial |
| Glaxosmithkline | TAGAMET | cimetidine hydrochloride | SOLUTION;ORAL | 017924-001 | Approved Prior to Jan 1, 1982 | 4,024,271 | ⤷ Try a Trial |
| Glaxosmithkline Llc | REQUIP XL | ropinirole hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 022008-006 | Apr 10, 2009 | 7,927,624 | ⤷ Try a Trial |
| Glaxosmithkline | IMITREX | sumatriptan | SPRAY;NASAL | 020626-001 | Aug 26, 1997 | 5,307,953*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for GLAXOSMITHKLINE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 25 mg, 50 mg, 100 mg, 200 mg, 250 mg, and 300 mg | ➤ Subscribe | 2014-02-12 |
| ➤ Subscribe | Extended-release Tablets | 8 mg | ➤ Subscribe | 2008-11-03 |
| ➤ Subscribe | Extended-release Tablets | 12 mg | ➤ Subscribe | 2009-02-05 |
| ➤ Subscribe | Extended-release Tablets | 6 mg | ➤ Subscribe | 2009-07-22 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2007-10-30 |
| ➤ Subscribe | Orally Disintegrating Tablets | 25 mg, 50 mg, 100 mg, and 200 mg | ➤ Subscribe | 2009-12-21 |
| ➤ Subscribe | Injection | 6 mg/0.5 mL, 0.5 mL (prefilled syringes) | ➤ Subscribe | 2006-05-09 |
| ➤ Subscribe | Tablets | 3 mg, 4 mg and 5 mg | ➤ Subscribe | 2005-02-04 |
| ➤ Subscribe | Tablets | 250 mg/100 mg | ➤ Subscribe | 2009-04-03 |
| ➤ Subscribe | Extended-release Capsules | 325 mg | ➤ Subscribe | 2006-11-07 |
| ➤ Subscribe | Oral Suspension | 750 mg/5 mL | ➤ Subscribe | 2009-10-20 |
| ➤ Subscribe | Extended-release Tablets | 4 mg | ➤ Subscribe | 2008-10-31 |
| ➤ Subscribe | Extended-release Tablets | 3 mg | ➤ Subscribe | 2009-01-08 |
| ➤ Subscribe | Extended-release Tablets | 2 mg | ➤ Subscribe | 2008-10-14 |
| ➤ Subscribe | Extended-release Tablets | 3 mg | ➤ Subscribe | 2009-01-08 |
| ➤ Subscribe | Injection | 6 mg/0.5 mL, 0.5 mL vials | ➤ Subscribe | 2004-10-25 |
| ➤ Subscribe | Tablets | 0.25 mg, 0.5 mg, 1 mg and 2 mg | ➤ Subscribe | 2004-12-22 |
| ➤ Subscribe | Tablets | 100 mg | ➤ Subscribe | 2007-10-31 |
| ➤ Subscribe | Tablets | 62.5 mg/25 mg | ➤ Subscribe | 2010-09-14 |
| ➤ Subscribe | Extended-release Capsules | 225 mg and 425 mg | ➤ Subscribe | 2006-10-11 |
International Patents for Glaxosmithkline Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| United Kingdom | 0201677 | ⤷ Try a Trial |
| China | 1856572 | ⤷ Try a Trial |
| New Zealand | 548888 | ⤷ Try a Trial |
| Canada | 2674436 | ⤷ Try a Trial |
| United Kingdom | 2419882 | ⤷ Try a Trial |
| Cyprus | 1114641 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Glaxosmithkline Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2924034 | 300981 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DORAVIRINE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT IN COMBINATIE MET LAMIVUDINE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN COMBINATIE MET TENOFOVIR OF EEN ESTER DAARVAN, IN HET BIJZONDER EEN DISOPROXIL ESTER, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER EEN FUMARAAT ZOUT; REGISTRATION NO/DATE: EU/1/18/1333/001-002 20181126 |
| 1425001 | PA2014019 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: VILANTEROLUM; REGISTRATION NO/DATE: EU/1/13/886/001-006 20131113 |
| 1425001 | 1490033-6 | Sweden | ⤷ Try a Trial | PRODUCT NAME: VILANTEROL, OCH SALTER OCH SOLVATER DAERAV; REG. NO/DATE: EU/1/13/886 20131113 |
| 2240466 | 122018000052 | Germany | ⤷ Try a Trial | PRODUCT NAME: NIRAPARIBTOSYLAT, EINSCHLIESSLICH NIRAPARIBTOSYLATMONOHYDRAT; REGISTRATION NO/DATE: EU/1/17/1235 20171116 |
| 1633724 | 92680 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: OLAPARIB AINSI QUE DES SELS ET DES SOLVATES DE CELUI-CI. FIRST REGISTRATION: 20141218 |
| 1425001 | C300664 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: VILANTEROL, DAN WEL EEN ZOUT OF SOLVAAT DAARVAN, IN HET BIJZONDER HET TRIFENYLACETAATZOUT; REGISTRATION NO/DATE: EU/1/13/886/001-006 20131113 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.