GILEAD SCIENCES Company Profile
✉ Email this page to a colleague
What is the competitive landscape for GILEAD SCIENCES, and when can generic versions of GILEAD SCIENCES drugs launch?
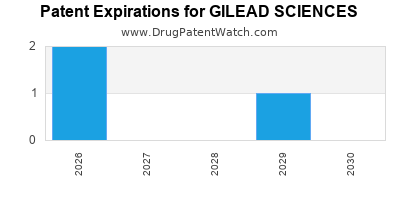
GILEAD SCIENCES has twenty-four approved drugs.
There are eighty-five US patents protecting GILEAD SCIENCES drugs.
There are two thousand two hundred and eighty-one patent family members on GILEAD SCIENCES drugs in sixty-six countries and three hundred and seventy-two supplementary protection certificates in nineteen countries.
Summary for GILEAD SCIENCES
| International Patents: | 2281 |
| US Patents: | 85 |
| Tradenames: | 19 |
| Ingredients: | 19 |
| NDAs: | 24 |
| Patent Litigation for GILEAD SCIENCES: | See patent lawsuits for GILEAD SCIENCES |
| PTAB Cases with GILEAD SCIENCES as patent owner: | See PTAB cases with GILEAD SCIENCES as patent owner |
Drugs and US Patents for GILEAD SCIENCES
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | VOSEVI | sofosbuvir; velpatasvir; voxilaprevir | TABLET;ORAL | 209195-001 | Jul 18, 2017 | RX | Yes | Yes | 7,964,580*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Gilead Sciences Inc | HARVONI | ledipasvir; sofosbuvir | TABLET;ORAL | 205834-002 | Aug 28, 2019 | RX | Yes | No | 7,964,580*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Gilead Sciences Inc | HARVONI | ledipasvir; sofosbuvir | TABLET;ORAL | 205834-001 | Oct 10, 2014 | RX | Yes | Yes | 8,889,159*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Gilead Sciences Inc | EPCLUSA | sofosbuvir; velpatasvir | PELLETS;ORAL | 214187-002 | Jun 10, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for GILEAD SCIENCES
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | COMPLERA | emtricitabine; rilpivirine hydrochloride; tenofovir disoproxil fumarate | TABLET;ORAL | 202123-001 | Aug 10, 2011 | 8,592,397 | ⤷ Try a Trial |
| Gilead Sciences Inc | VIREAD | tenofovir disoproxil fumarate | TABLET;ORAL | 021356-001 | Oct 26, 2001 | 6,057,305 | ⤷ Try a Trial |
| Gilead Sciences Inc | VIREAD | tenofovir disoproxil fumarate | TABLET;ORAL | 021356-002 | Jan 18, 2012 | 5,977,089*PED | ⤷ Try a Trial |
| Gilead Sciences Inc | STRIBILD | cobicistat; elvitegravir; emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 203100-001 | Aug 27, 2012 | 6,642,245*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for GILEAD SCIENCES drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2010-01-26 |
| ➤ Subscribe | Tablets | 200 mg/25 mg/300 mg | ➤ Subscribe | 2015-05-20 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2015-12-09 |
| ➤ Subscribe | Tablets | 600 mg/200 mg/300 mg | ➤ Subscribe | 2008-12-29 |
| ➤ Subscribe | Tablets | 150 mg, 200 mg, and 250 mg | ➤ Subscribe | 2012-05-17 |
| ➤ Subscribe | Tablets | 150 mg, 150 mg, 200 mg, 300 mg | ➤ Subscribe | 2018-10-04 |
International Patents for GILEAD SCIENCES Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Poland | 2552930 | ⤷ Try a Trial |
| Norway | 2017066 | ⤷ Try a Trial |
| South Korea | 101759369 | ⤷ Try a Trial |
| Lithuania | C3150586 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for GILEAD SCIENCES Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2937350 | C20200043 00387 | Estonia | ⤷ Try a Trial | PRODUCT NAME: REMDESIVIIR;REG NO/DATE: EU/1/20/1459 03.07.2020 |
| 2635588 | C02635588/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: VELPATASVIR + SOFOSBUVIR + VOXILAPREVIR; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 66510 08.12.2017 |
| 2924034 | 2019/021 | Ireland | ⤷ Try a Trial | PRODUCT NAME: DORAVIRINE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF IN COMBINATION WITH LAMIVUDINE AND TENOFOVIR DISOPROXIL FUMARATE; REGISTRATION NO/DATE: EU/1/18/1333/001 EU/1/18/1333/002 20181122 |
| 1564210 | PA2013018 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: ELVITEGRAVIRUM; REGISTRATION NO/DATE: EU/1/13/830/001, 2013 05 24 EU/1/13/830/002 20130524 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.