GALDERMA LABS LP Company Profile
✉ Email this page to a colleague
What is the competitive landscape for GALDERMA LABS LP, and when can generic versions of GALDERMA LABS LP drugs launch?
GALDERMA LABS LP has twenty-six approved drugs.
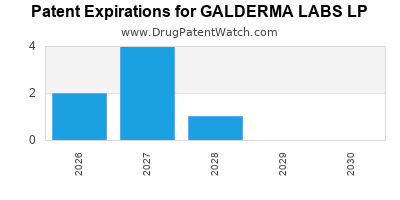
There are fifty-eight US patents protecting GALDERMA LABS LP drugs.
There are four hundred and four patent family members on GALDERMA LABS LP drugs in thirty-seven countries and one hundred and seventy-seven supplementary protection certificates in eighteen countries.
Summary for GALDERMA LABS LP
| International Patents: | 404 |
| US Patents: | 58 |
| Tradenames: | 18 |
| Ingredients: | 16 |
| NDAs: | 26 |
Drugs and US Patents for GALDERMA LABS LP
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | AKLIEF | trifarotene | CREAM;TOPICAL | 211527-001 | Oct 4, 2019 | RX | Yes | Yes | 9,084,778 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Galderma Labs Lp | TRI-LUMA | fluocinolone acetonide; hydroquinone; tretinoin | CREAM;TOPICAL | 021112-001 | Jan 18, 2002 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Galderma Labs Lp | MIRVASO | brimonidine tartrate | GEL;TOPICAL | 204708-001 | Aug 23, 2013 | AB | RX | Yes | Yes | 8,231,885 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Galderma Labs Lp | TWYNEO | benzoyl peroxide; tretinoin | CREAM;TOPICAL | 214902-001 | Jul 26, 2021 | RX | Yes | Yes | 10,420,743 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Galderma Labs Lp | DIFFERIN | adapalene | GEL;TOPICAL | 021753-001 | Jun 19, 2007 | AB | RX | Yes | Yes | 7,579,377 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for GALDERMA LABS LP
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Galderma Labs Lp | ORACEA | doxycycline | CAPSULE;ORAL | 050805-001 | May 26, 2006 | 5,919,775 | ⤷ Try a Trial |
| Galderma Labs Lp | ORACEA | doxycycline | CAPSULE;ORAL | 050805-001 | May 26, 2006 | 7,211,267 | ⤷ Try a Trial |
| Galderma Labs Lp | CLOBEX | clobetasol propionate | SPRAY;TOPICAL | 021835-001 | Oct 27, 2005 | 5,990,100 | ⤷ Try a Trial |
| Galderma Labs Lp | ORACEA | doxycycline | CAPSULE;ORAL | 050805-001 | May 26, 2006 | 7,232,572 | ⤷ Try a Trial |
| Galderma Labs Lp | METROGEL | metronidazole | GEL;TOPICAL | 019737-001 | Nov 22, 1988 | 4,837,378 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for GALDERMA LABS LP drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Topical Gel | 0.30% | ➤ Subscribe | 2009-09-15 |
| ➤ Subscribe | Gel | 0.1%/2.5% | ➤ Subscribe | 2011-12-30 |
| ➤ Subscribe | Topical Gel | 0.33% | ➤ Subscribe | 2014-12-15 |
| ➤ Subscribe | Spray | 0.05% | ➤ Subscribe | 2008-09-29 |
| ➤ Subscribe | Delayed-release Capsules | 40 mg | ➤ Subscribe | 2008-12-11 |
| ➤ Subscribe | Cream | 1% | ➤ Subscribe | 2016-12-30 |
| ➤ Subscribe | Topical Gel | 1% | ➤ Subscribe | 2008-10-21 |
| ➤ Subscribe | Lotion | 0.05% | ➤ Subscribe | 2006-03-27 |
| ➤ Subscribe | Topical Shampoo | 0.05% | ➤ Subscribe | 2008-01-09 |
International Patents for GALDERMA LABS LP Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Australia | 2013269582 | ⤷ Try a Trial |
| Poland | 220838 | ⤷ Try a Trial |
| South Korea | 101291824 | ⤷ Try a Trial |
| Slovenia | 1761266 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 03075908 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for GALDERMA LABS LP Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2435024 | 21C1020 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE FORMOTEROL (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI), GLYCOPYRROLATE (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI) ET BUDESONIDE (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI); REGISTRATION NO/DATE: EU/1/20/1498 20201210 |
| 0473687 | SPC/GB98/030 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: FOSPHENYTOIN SODIUM (3-HYDROXYMETHYL)-5,5-DIPHENYLHYDANTOIN DISODIUM PHOSPHATE ESTER); REGISTERED: UK 0019/0157 19980204 |
| 0820432 | SPC/GB02/038 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: METHYL AMINOLEVULINATE, OPTIONALLY IN THE FORM OF A SALT, PREFERABLY METHYL AMINOLEVULINATE HYDROCHLORIDE; REGISTERED: SE 16338 20010615; UK PL18952/0002 20020408 |
| 2826776 | LUC00207 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF (A) (R)-1-(2,2-DIFLUOROBENZO(D)(1,3)DIOXOL-5-YL)-N-(1-(2,3-DIHYDROXYPROPYL)-6-FLUORO-2-(1-HYDROXY-2-METHYLPROPAN-2-YL)-1H-INDOL-5-YL)CYCLOPROPANECARBOXAMIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF AND (B) N-(5-HYDROXY-2,4-DITERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLINE-3-CARBOXAMIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; AUTHORISATION NUMBER AND DATE: EU/1/18/1306 20181106 |
| 2826776 | CR 2021 00013 | Denmark | ⤷ Try a Trial | PRODUCT NAME: EN KOMBINATION AF (A) (R)-1-(2,2-DIFLUOROBENZO(D)(1,3)DIOXOL-5-YL)-N-(1-(2,3-DIHYDROXYPROPYL)-6-FLUORO-2-(1-HYDROXY-2-METHYLPROPAN-2-YL)-1H-INDOL-5-YL)CYCLOPROPANECARBOXAMID ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF OG (B) ....; REG. NO/DATE: EU/1/18/1306 20181106 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.