FERRING Company Profile
✉ Email this page to a colleague
What is the competitive landscape for FERRING, and when can generic versions of FERRING drugs launch?
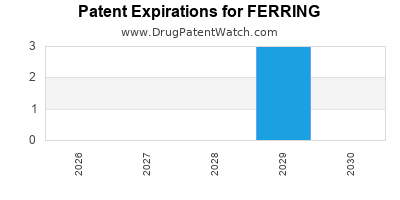
FERRING has seventeen approved drugs.
There are nineteen US patents protecting FERRING drugs.
There are two hundred and seventy-six patent family members on FERRING drugs in forty countries and twenty-one supplementary protection certificates in ten countries.
Summary for FERRING
| International Patents: | 276 |
| US Patents: | 19 |
| Tradenames: | 17 |
| Ingredients: | 8 |
| NDAs: | 17 |
| Patent Litigation for FERRING: | See patent lawsuits for FERRING |
Drugs and US Patents for FERRING
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferring | FIRMAGON | degarelix acetate | POWDER;SUBCUTANEOUS | 022201-001 | Dec 24, 2008 | RX | Yes | No | 11,766,468 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Ferring Pharms Inc | MILPROSA | progesterone | SYSTEM;VAGINAL | 201110-001 | Apr 29, 2020 | DISCN | Yes | No | 8,580,293 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Ferring Pharms Inc | CLENPIQ | citric acid; magnesium oxide; sodium picosulfate | SOLUTION;ORAL | 209589-001 | Nov 28, 2017 | RX | Yes | Yes | 9,827,231 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Ferring | DESMOPRESSIN ACETATE | desmopressin acetate | TABLET;ORAL | 021795-002 | May 8, 2008 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Ferring Pharms Inc | CLENPIQ | citric acid; magnesium oxide; sodium picosulfate | SOLUTION;ORAL | 209589-001 | Nov 28, 2017 | RX | Yes | Yes | 11,191,753 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Ferring | DESMOPRESSIN ACETATE | desmopressin acetate | TABLET;ORAL | 021795-001 | May 8, 2008 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for FERRING
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ferring Pharms Inc | NOCDURNA | desmopressin acetate | TABLET;SUBLINGUAL | 022517-001 | Jun 21, 2018 | 8,802,624 | ⤷ Try a Trial |
| Ferring | ENDOMETRIN | progesterone | INSERT;VAGINAL | 022057-001 | Jun 21, 2007 | 7,320,800 | ⤷ Try a Trial |
| Ferring Pharms Inc | DDAVP | desmopressin acetate | TABLET;ORAL | 019955-002 | Sep 6, 1995 | 7,022,340 | ⤷ Try a Trial |
| Ferring | ENDOMETRIN | progesterone | INSERT;VAGINAL | 022057-001 | Jun 21, 2007 | 7,393,543 | ⤷ Try a Trial |
| Ferring Pharms Inc | NOCDURNA | desmopressin acetate | TABLET;SUBLINGUAL | 022517-002 | Jun 21, 2018 | 9,504,647 | ⤷ Try a Trial |
| Ferring | FIRMAGON | degarelix acetate | POWDER;SUBCUTANEOUS | 022201-001 | Dec 24, 2008 | 5,925,730 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for FERRING drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Oral Solution | 10 mg, 3.5 g, and12 g | ➤ Subscribe | 2019-02-11 |
| ➤ Subscribe | Oral Solution | 10 mg, 3.5 g, and 12 g | ➤ Subscribe | 2014-05-21 |
International Patents for FERRING Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 102098991 | ⤷ Try a Trial |
| Norway | 335167 | ⤷ Try a Trial |
| Uruguay | 27683 | ⤷ Try a Trial |
| Denmark | 3006036 | ⤷ Try a Trial |
| Spain | 2648640 | ⤷ Try a Trial |
| Japan | 2018035198 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for FERRING Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2712622 | LUC00015 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: DESMOPRESSINE OU UN DE SES SELS D'ACETATE; AUTHORISATION NUMBER AND DATE: 497271; 497280 20161101 |
| 1003774 | PA2009005,C1003774 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: DEGARELIXUM ACETAT; REGISTRATION NO/DATE: EU/1/08/504/001, 2009 02 17 EU/1/08/504/002 20090217 |
| 3225249 | 300983 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DESMOPRESSIN, DESGEWENST IN DE VORM VAN EEN ACETAAT ZOUT; REGISTRATION NO/DATE: BE497271 & BE497280 20160513 |
| 0113964 | 97C0037 | Belgium | ⤷ Try a Trial | PRODUCT NAME: OESTROGENES EQUINS CONJUGUES; ACETATE DE MEDROXYPROGESTERONE; NAT. REGISTRATION NO/DATE: NL 19569 19950301; FIRST REGISTRATION: CH - 52647 01 010 19940826 |
| 2782584 | 301153 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTAINING BOTH ESTRADIOL (17SS-ESTRADIOL), OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE OR SOLVATE THEREOF (INCLUDING IN HEMIHYDRATE FORM), AND PROGESTERONE; NATIONAL REGISTRATION NO/DATE: RVG 125821 20210611; FIRST REGISTRATION: BE BE582231 20210406 |
| 1003774 | PA2009005 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: DEGARELIXUM ACETAT; REGISTRATION NO/DATE: EU/1/08/504/001, 2009 02 17 EU/1/08/504/002 20090217 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.