Daiichi Sankyo Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for DAIICHI SANKYO INC, and what generic alternatives to DAIICHI SANKYO INC drugs are available?
DAIICHI SANKYO INC has three approved drugs.
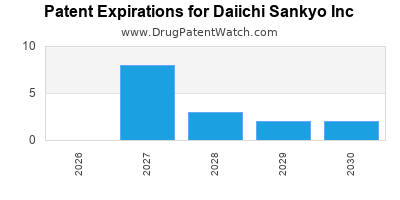
There are twenty-five US patents protecting DAIICHI SANKYO INC drugs.
There are three hundred and nineteen patent family members on DAIICHI SANKYO INC drugs in forty-five countries and seventeen supplementary protection certificates in fifteen countries.
Summary for Daiichi Sankyo Inc
| International Patents: | 319 |
| US Patents: | 25 |
| Tradenames: | 3 |
| Ingredients: | 3 |
| NDAs: | 3 |
Drugs and US Patents for Daiichi Sankyo Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daiichi Sankyo Inc | VANFLYTA | quizartinib dihydrochloride | TABLET;ORAL | 216993-001 | Jul 20, 2023 | RX | Yes | No | 8,883,783 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Daiichi Sankyo Inc | TURALIO | pexidartinib hydrochloride | CAPSULE;ORAL | 211810-001 | Aug 2, 2019 | DISCN | Yes | No | 8,404,700 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Daiichi Sankyo Inc | TURALIO | pexidartinib hydrochloride | CAPSULE;ORAL | 211810-001 | Aug 2, 2019 | DISCN | Yes | No | 10,961,240 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Paragraph IV (Patent) Challenges for DAIICHI SANKYO INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 15 mg, 30 mg and 60 mg | ➤ Subscribe | 2019-01-28 |
International Patents for Daiichi Sankyo Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| South Korea | 20150105494 | ⤷ Try a Trial |
| Australia | 2010248860 | ⤷ Try a Trial |
| Singapore | 179497 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Daiichi Sankyo Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2140867 | 292 50001-2018 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: EDOXABAN VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/15/993 20150623 |
| 1405852 | CR 2015 00052 | Denmark | ⤷ Try a Trial | PRODUCT NAME: EDOXABAN, OR A SALT THEREOF, A SOLVATE THEREOF, OR AN N-OXIDE THEREOF, IN PARTICULAR EDOXABAN TOSYLATE; REG. NO/DATE: EU/1/15/993/001-028 20150623 |
| 1405852 | 300760 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: EDOXABAN, EEN ZOUT DAARVAN, EEN SOLVAAT DAARVAAN OF EEN N-OXIDE DAARVAN, IN HET BIJZONDER EDOXABAN TOSYLAAT; REGISTRATION NO/DATE: EU/1/15/993 20150619 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.