CURRAX Company Profile
✉ Email this page to a colleague
What is the competitive landscape for CURRAX, and what generic alternatives to CURRAX drugs are available?
CURRAX has three approved drugs.
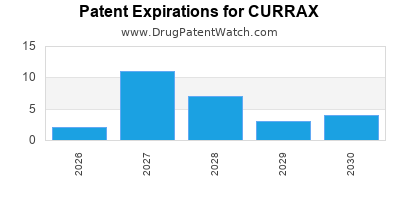
There are thirty-two US patents protecting CURRAX drugs.
There are two hundred and seventy-nine patent family members on CURRAX drugs in twenty-nine countries and twelve supplementary protection certificates in nine countries.
Drugs and US Patents for CURRAX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Currax | SILENOR | doxepin hydrochloride | TABLET;ORAL | 022036-002 | Mar 17, 2010 | AB | RX | Yes | Yes | 10,548,871 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Currax | ONZETRA XSAIL | sumatriptan succinate | POWDER;NASAL | 206099-001 | Jan 27, 2016 | RX | Yes | Yes | 10,076,614 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Currax | ONZETRA XSAIL | sumatriptan succinate | POWDER;NASAL | 206099-001 | Jan 27, 2016 | RX | Yes | Yes | 10,722,667 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Currax | ONZETRA XSAIL | sumatriptan succinate | POWDER;NASAL | 206099-001 | Jan 27, 2016 | RX | Yes | Yes | 11,571,531 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Currax | SILENOR | doxepin hydrochloride | TABLET;ORAL | 022036-002 | Mar 17, 2010 | AB | RX | Yes | Yes | 9,107,898 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Currax | ONZETRA XSAIL | sumatriptan succinate | POWDER;NASAL | 206099-001 | Jan 27, 2016 | RX | Yes | Yes | 10,076,615 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Currax | SILENOR | doxepin hydrochloride | TABLET;ORAL | 022036-001 | Mar 17, 2010 | AB | RX | Yes | No | 11,110,074 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for CURRAX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Currax | TREXIMET | naproxen sodium; sumatriptan succinate | TABLET;ORAL | 021926-001 | Apr 15, 2008 | 5,037,845 | ⤷ Try a Trial |
| Currax | SILENOR | doxepin hydrochloride | TABLET;ORAL | 022036-002 | Mar 17, 2010 | 5,725,884 | ⤷ Try a Trial |
| Currax | TREXIMET | naproxen sodium; sumatriptan succinate | TABLET;ORAL | 021926-001 | Apr 15, 2008 | 8,022,095*PED | ⤷ Try a Trial |
| Currax | ONZETRA XSAIL | sumatriptan succinate | POWDER;NASAL | 206099-001 | Jan 27, 2016 | 8,327,844 | ⤷ Try a Trial |
| Currax | ONZETRA XSAIL | sumatriptan succinate | POWDER;NASAL | 206099-001 | Jan 27, 2016 | 8,555,877 | ⤷ Try a Trial |
| Currax | TREXIMET | naproxen sodium; sumatriptan succinate | TABLET;ORAL | 021926-002 | May 14, 2015 | 6,060,499*PED | ⤷ Try a Trial |
| Currax | SILENOR | doxepin hydrochloride | TABLET;ORAL | 022036-002 | Mar 17, 2010 | 6,217,909 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for CURRAX drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 3 mg and 6 mg | ➤ Subscribe | 2010-09-16 |
International Patents for CURRAX Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| United Kingdom | 2378393 | ⤷ Try a Trial |
| Australia | 2007202918 | ⤷ Try a Trial |
| Germany | 60017099 | ⤷ Try a Trial |
| United Kingdom | 2391483 | ⤷ Try a Trial |
| United Kingdom | 0503738 | ⤷ Try a Trial |
| Austria | 422923 | ⤷ Try a Trial |
| South Africa | 200107206 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for CURRAX Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1411900 | SPC/GB11/015 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: NAPROXEN AND ESOMEPRAZOLE; REGISTERED: UK PL 17901/0263-0001 20101105 |
| 1411900 | 2011/016 | Ireland | ⤷ Try a Trial | PRODUCT NAME: NAPROXEN AND ESOMEPRAZOLE MODIFIED-RELEASE TABLETS; NAT REGISTRATION NO/DATE: PA0970/060/001 20101221; FIRST REGISTRATION NO/DATE: PL17901/0263-0001 20101105 |
| 1411900 | 122012000052 | Germany | ⤷ Try a Trial | PRODUCT NAME: NAPROXEN MIT ESOMEPRAZOL; NAT. REGISTRATION NO/DATE: 85145.00.00 20120202; FIRST REGISTRATION: GROSSBRITANNIEN PL 17901/0263 - 0001 20101105 |
| 0984957 | PA2011005,C0984957 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: NAPROXENUM + ESOMEPRAZOLUM; REGISTRATION NO/DATE: LT/1/10/2302/001-LT/1/10/2302/012 20110126 |
| 1411900 | 18/2011 | Austria | ⤷ Try a Trial | PRODUCT NAME: NAPROXEN UND ESOMEPRAZOL SOWIE DEREN PHARMAZEUTISCH ANNEHMBARE SALZE; NAT. REGISTRATION NO/DATE: 1-29937 20110105; FIRST REGISTRATION: GB PL 17901/0263-0001 20101105 |
| 0984957 | SPC/GB11/013 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: NAPROXEN AND ESOMEPRAZOLE; REGISTERED: UK PL 17901/0263-0001 20101105 |
| 1411900 | C300481 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: NAPROXEN EN ESOMEPRAZOL; NAT. REGISTRATION NO/DATE: RVG 106235 20101118; FIRST REGISTRATION: PL 17901/0263-001 20101105 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.