CEPHALON Company Profile
✉ Email this page to a colleague
What is the competitive landscape for CEPHALON, and what generic alternatives to CEPHALON drugs are available?
CEPHALON has eight approved drugs.
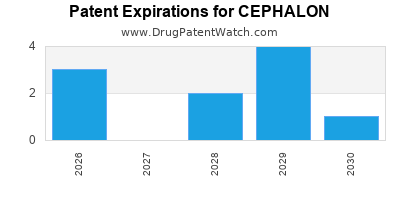
There are fifteen US patents protecting CEPHALON drugs.
There are two hundred and thirty-eight patent family members on CEPHALON drugs in thirty-five countries and fourteen supplementary protection certificates in five countries.
Summary for CEPHALON
| International Patents: | 238 |
| US Patents: | 15 |
| Tradenames: | 8 |
| Ingredients: | 6 |
| NDAs: | 8 |
| Patent Litigation for CEPHALON: | See patent lawsuits for CEPHALON |
| PTAB Cases with CEPHALON as patent owner: | See PTAB cases with CEPHALON as patent owner |
Drugs and US Patents for CEPHALON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cephalon | FENTORA | fentanyl citrate | TABLET;BUCCAL, SUBLINGUAL | 021947-003 | Sep 25, 2006 | RX | Yes | Yes | 8,119,158 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Cephalon | FENTANYL | fentanyl citrate | TROCHE/LOZENGE;ORAL | 020195-007 | Oct 30, 1995 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Cephalon | FENTORA | fentanyl citrate | TABLET;BUCCAL, SUBLINGUAL | 021947-001 | Sep 25, 2006 | RX | Yes | No | 7,862,833 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Cephalon | FENTORA | fentanyl citrate | TABLET;BUCCAL, SUBLINGUAL | 021947-004 | Sep 25, 2006 | RX | Yes | No | 8,119,158 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Cephalon | TREANDA | bendamustine hydrochloride | POWDER;IV (INFUSION) | 022249-001 | Mar 20, 2008 | AP | RX | Yes | Yes | 8,791,270*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Cephalon | NUVIGIL | armodafinil | TABLET;ORAL | 021875-001 | Jun 15, 2007 | AB | RX | Yes | No | 7,132,570*PED | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Cephalon | FENTORA | fentanyl citrate | TABLET;BUCCAL, SUBLINGUAL | 021947-005 | Sep 25, 2006 | RX | Yes | No | 8,092,832 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for CEPHALON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Cephalon | ACTIQ | fentanyl citrate | TROCHE/LOZENGE;TRANSMUCOSAL | 020747-006 | Nov 4, 1998 | 4,671,953 | ⤷ Try a Trial |
| Cephalon | FENTORA | fentanyl citrate | TABLET;BUCCAL, SUBLINGUAL | 021947-003 | Sep 25, 2006 | 8,753,611 | ⤷ Try a Trial |
| Cephalon | TRISENOX | arsenic trioxide | INJECTABLE;INJECTION | 021248-001 | Sep 25, 2000 | 6,861,076 | ⤷ Try a Trial |
| Cephalon | FENTORA | fentanyl citrate | TABLET;BUCCAL, SUBLINGUAL | 021947-006 | Mar 2, 2007 | 6,974,590 | ⤷ Try a Trial |
| Cephalon | TRISENOX | arsenic trioxide | INJECTABLE;INJECTION | 021248-001 | Sep 25, 2000 | 6,884,439 | ⤷ Try a Trial |
| Cephalon | ACTIQ | fentanyl citrate | TROCHE/LOZENGE;TRANSMUCOSAL | 020747-006 | Nov 4, 1998 | 4,863,737 | ⤷ Try a Trial |
| Cephalon | NUVIGIL | armodafinil | TABLET;ORAL | 021875-003 | Jun 15, 2007 | RE37516*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for CEPHALON drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 25 mg/vial and 100 mg/vial | ➤ Subscribe | 2013-06-04 |
| ➤ Subscribe | Buccal Tablets | 0.1 mg, 0.2 mg, 0.3 mg, 0.4 mg, 0.6 mg and 0.8 mg | ➤ Subscribe | 2007-11-13 |
| ➤ Subscribe | Tablets | 12 mg and 16 mg | ➤ Subscribe | 2014-01-24 |
| ➤ Subscribe | Tablets | 100 mg and 200 mg | ➤ Subscribe | 2009-09-11 |
| ➤ Subscribe | Lozenges | 0.4 mg | ➤ Subscribe | 2004-10-06 |
| ➤ Subscribe | Lozenges | 0.8 mg, 1.2 mg and 1.6 mg | ➤ Subscribe | 2004-11-22 |
| ➤ Subscribe | Injection | 1 mg/mL | ➤ Subscribe | 2015-08-11 |
| ➤ Subscribe | Injection | 90 mg/mL, 0.5 mL and 2 mL in single-dose vials | ➤ Subscribe | 2014-06-19 |
| ➤ Subscribe | Tablets | 2 mg and 4 mg | ➤ Subscribe | 2005-02-01 |
| ➤ Subscribe | Tablets | 50 mg, 150 mg and 250 mg | ➤ Subscribe | 2009-07-24 |
| ➤ Subscribe | Lozenges | 0.2 mg | ➤ Subscribe | 2004-10-29 |
| ➤ Subscribe | Lozenges | 0.6 mg | ➤ Subscribe | 2004-12-20 |
International Patents for CEPHALON Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Malaysia | 157661 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2009120386 | ⤷ Try a Trial |
| European Patent Office | 1556026 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2010036702 | ⤷ Try a Trial |
| South Korea | 101125361 | ⤷ Try a Trial |
| Japan | 2013056902 | ⤷ Try a Trial |
| Spain | 2359979 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for CEPHALON Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0836511 | 122006000022 | Germany | ⤷ Try a Trial | PRODUCT NAME: TRANSDERMAL IONTOPHORETISCH VERABREICHTES FENTANYL-HYDROCHLORID; REGISTRATION NO/DATE: EU/1/05/326/001 20060126 |
| 1635783 | 300653 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: FENTANYL IN ELKE DOOR HET BASISOCTROOI BESCHERMDE VERSCHIJNINGSVORM; REGISTRATION NO/DATE: EU/1/10/644/001-004 20100906 |
| 0836511 | SPC/GB06/022 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: FENTANYL HYDROCHLORIDE; REGISTERED: UK EU/1/05/326/001 20060124 |
| 0236342 | 97C0107 | Belgium | ⤷ Try a Trial | PRODUCT NAME: TIAGABINE CHLORHYDRATE MONOHYDRATE - TIAGABINE ANHYDRE; NAT. REGISTRATION NO/DATE: 403 IS 102 F 3 19970602; FIRST REGISTRATION: FR 341 260.2 19960614 |
| 0975367 | 122011000009 | Germany | ⤷ Try a Trial | PRODUCT NAME: FENTANYL IN ALLEN DEM SCHUTZ DES GRUNDPATENTS UNTERLIEGENDEN FORMEN; REGISTRATION NO/DATE: EU/1/10/644/001-004 20100831 |
| 0901368 | C300523 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: FENTANYL; REGISTRATION NO/DATE: EU/2/11/127/001 20111006 |
| 1635783 | C300653 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: FENTANYL IN ELKE DOOR HET BASISOCTROOI BESCHERMDE VERSCHIJNINGSVORM; REGISTRATION NO/DATE: EU/1/10/644/001-004 20100906 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.