BRISTOL-MYERS SQUIBB Company Profile
✉ Email this page to a colleague
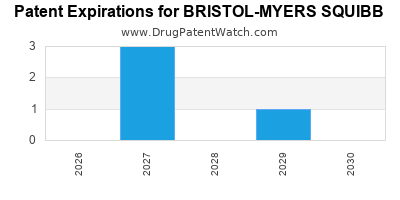
What is the competitive landscape for BRISTOL-MYERS SQUIBB, and when can generic versions of BRISTOL-MYERS SQUIBB drugs launch?
BRISTOL-MYERS SQUIBB has one approved drug.
There are five US patents protecting BRISTOL-MYERS SQUIBB drugs.
There are eighty-one patent family members on BRISTOL-MYERS SQUIBB drugs in thirty-one countries and nineteen supplementary protection certificates in sixteen countries.
Summary for BRISTOL-MYERS SQUIBB
| International Patents: | 81 |
| US Patents: | 5 |
| Tradenames: | 1 |
| Ingredients: | 1 |
| NDAs: | 1 |
| Patent Litigation for BRISTOL-MYERS SQUIBB: | See patent lawsuits for BRISTOL-MYERS SQUIBB |
| PTAB Cases with BRISTOL-MYERS SQUIBB as patent owner: | See PTAB cases with BRISTOL-MYERS SQUIBB as patent owner |
Drugs and US Patents for BRISTOL-MYERS SQUIBB
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bristol-myers Squibb | DAKLINZA | daclatasvir dihydrochloride | TABLET;ORAL | 206843-001 | Jul 24, 2015 | DISCN | Yes | No | 8,329,159 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Bristol-myers Squibb | DAKLINZA | daclatasvir dihydrochloride | TABLET;ORAL | 206843-001 | Jul 24, 2015 | DISCN | Yes | No | 8,900,566 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bristol-myers Squibb | DAKLINZA | daclatasvir dihydrochloride | TABLET;ORAL | 206843-002 | Jul 24, 2015 | DISCN | Yes | No | 8,900,566 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bristol-myers Squibb | DAKLINZA | daclatasvir dihydrochloride | TABLET;ORAL | 206843-002 | Jul 24, 2015 | DISCN | Yes | No | 9,421,192 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Bristol-myers Squibb | DAKLINZA | daclatasvir dihydrochloride | TABLET;ORAL | 206843-001 | Jul 24, 2015 | DISCN | Yes | No | 8,642,025 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for BRISTOL-MYERS SQUIBB Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 2660520 | ⤷ Try a Trial |
| Hong Kong | 1201535 | ⤷ Try a Trial |
| New Zealand | 574805 | ⤷ Try a Trial |
| Croatia | P20160410 | ⤷ Try a Trial |
| European Patent Office | 2183244 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for BRISTOL-MYERS SQUIBB Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2049522 | 300713 | Netherlands | ⤷ Try a Trial | DETAILS ASSIGNMENT: CHANGE OF OWNER(S), CHANGE OF OWNER(S) NAME |
| 2049522 | C300713 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DACLATASVIR EN FARMACEUTISCH; REGISTRATION NO/DATE: EU/1/14/939/001-004 20150119 |
| 2049522 | CR 2015 00003 | Denmark | ⤷ Try a Trial | PRODUCT NAME: DACLATASVIR OG FARMACEUTISK ACCEPTABLE SALTE DERAF, SAERLIGT DACLATASVIR-DIHYDROCHLORID; REG. NO/DATE: EU/1/14/939 20140826 |
| 2049522 | 1590007-9 | Sweden | ⤷ Try a Trial | PRODUCT NAME: DACLATASVIR AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, ESPECIALLY DACLATASVIR DIHYDROCHLORIDE; REG. NO/DATE: EU/1/14/939 20140826 |
| 2049522 | 211 50001-2015 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: DAKLATASVIR DIHYDROCHLORID; REGISTRATION NO/DATE: EU/1/14/939 - EU/1/14/939/004 20140826 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.