BOEHRINGER INGELHEIM Company Profile
✉ Email this page to a colleague
What is the competitive landscape for BOEHRINGER INGELHEIM, and what generic alternatives to BOEHRINGER INGELHEIM drugs are available?
BOEHRINGER INGELHEIM has forty-eight approved drugs.
There are sixty-nine US patents protecting BOEHRINGER INGELHEIM drugs.
There are one thousand seven hundred and eighty-two patent family members on BOEHRINGER INGELHEIM drugs in sixty-three countries and two hundred and seventeen supplementary protection certificates in nineteen countries.
Summary for BOEHRINGER INGELHEIM
| International Patents: | 1782 |
| US Patents: | 69 |
| Tradenames: | 37 |
| Ingredients: | 29 |
| NDAs: | 48 |
| Patent Litigation for BOEHRINGER INGELHEIM: | See patent lawsuits for BOEHRINGER INGELHEIM |
| PTAB Cases with BOEHRINGER INGELHEIM as petitioner: | See PTAB cases with BOEHRINGER INGELHEIM as petitioner |
Drugs and US Patents for BOEHRINGER INGELHEIM
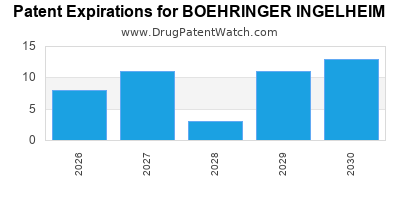
Expired US Patents for BOEHRINGER INGELHEIM
Paragraph IV (Patent) Challenges for BOEHRINGER INGELHEIM drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 0.125 mg, 0.5 mg, 1 mg and 1.5 mg | ➤ Subscribe | 2005-06-24 |
| ➤ Subscribe | Capsules | eq. to 75 mg base and 150 mg base | ➤ Subscribe | 2014-10-20 |
| ➤ Subscribe | Tablets | 0.75 mg | ➤ Subscribe | 2008-07-31 |
| ➤ Subscribe | Inhalation Powder Capsules | 18 mcg | ➤ Subscribe | 2018-05-11 |
| ➤ Subscribe | Tablets | 5 mg/500 mg5 mg/1000 mg12.5 mg/500 mg12.5 mg/1000 mg | ➤ Subscribe | 2018-08-01 |
| ➤ Subscribe | Extended-release Tablets | 0.375 mg, 0.75 mg, 1.5 mg, 3 mg and 4.5 mg | ➤ Subscribe | 2010-06-01 |
| ➤ Subscribe | Extended-release Tablets | 5 mg/1000 mg10 mg/1000 mg12.5 mg/1000 mg25 mg/1000 mg | ➤ Subscribe | 2018-08-01 |
| ➤ Subscribe | Tablets | 20 mg, 30 mg and 40 mg | ➤ Subscribe | 2017-07-12 |
| ➤ Subscribe | Tablets | 10 mg and 25 mg | ➤ Subscribe | 2018-08-01 |
| ➤ Subscribe | Tablets | 20 mg, 40 mg and 80 mg | ➤ Subscribe | 2006-12-26 |
| ➤ Subscribe | Tablets | 80 mg/25 mg | ➤ Subscribe | 2009-02-27 |
| ➤ Subscribe | Capsules | 100 mg and 150 mg | ➤ Subscribe | 2018-10-15 |
| ➤ Subscribe | Tablets | 0.25 mg | ➤ Subscribe | 2005-05-27 |
| ➤ Subscribe | Capsules | eq. to 110 mg base | ➤ Subscribe | 2015-12-15 |
| ➤ Subscribe | Oral Suspension | 7.5 mg/5 mL | ➤ Subscribe | 2009-12-17 |
| ➤ Subscribe | Extended-releaseTablets | 2.5 mg/1000 mg 5 mg/1000 mg | ➤ Subscribe | 2018-03-28 |
| ➤ Subscribe | Tablets | 5 mg | ➤ Subscribe | 2015-05-04 |
| ➤ Subscribe | Extended-release Tablets | 2.25 mg and 3.75 mg | ➤ Subscribe | 2011-07-26 |
| ➤ Subscribe | Extended-release Tablets | 400 mg | ➤ Subscribe | 2013-06-21 |
| ➤ Subscribe | Tablets | 10 mg/5 mg and 25 mg/5 mg | ➤ Subscribe | 2018-08-01 |
| ➤ Subscribe | Tablets | 2.5 mg/500 mg, 2.5 mg/850 mg, 2.5 mg/1000 mg | ➤ Subscribe | 2015-05-04 |
| ➤ Subscribe | Tablets | 80 mg/12.5 mg and 40 mg/12.5 mg | ➤ Subscribe | 2008-12-31 |
| ➤ Subscribe | Extended-release Capsules | 25 mg and 200 mg | ➤ Subscribe | 2007-02-01 |
International Patents for BOEHRINGER INGELHEIM Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2004053362 | ⤷ Try a Trial |
| Denmark | 2525812 | ⤷ Try a Trial |
| Israel | 209054 | ⤷ Try a Trial |
| Ukraine | 110470 | ⤷ Try a Trial |
| Ecuador | SP055569 | ⤷ Try a Trial |
| Peru | 20080698 | ⤷ Try a Trial |
| Japan | 4785847 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for BOEHRINGER INGELHEIM Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1562603 | 513 | Finland | ⤷ Try a Trial | |
| 1730131 | C20140033 00134 | Estonia | ⤷ Try a Trial | PRODUCT NAME: EMPAGLIFLOSIIN;REG NO/DATE: EU/1/14/930 27.05.2014 |
| 1507558 | 2012/018 | Ireland | ⤷ Try a Trial | PRODUCT NAME: ALISKIREN OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, AMLODIPINE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF AND HYDROCHLOROTHIAZIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF.; NAT REGISTRATION NO/DATE: EU/1/11/730/001-060 20111122; FIRST REGISTRATION NO/DATE: SWITZERLAND 6167801-6167805 20110705 |
| 1562603 | 300650 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: OLODATEROL, HET R-ENANTIOMEER DAARVAN, MENGSELS VAN ISOMEREN DAARVAN, ZUURADDITIEZOUTEN MET FARMACOLOGISCH AANVAARDBARE ZUREN DAARVAN, EVENALS SOLVATEN EN/OF HYDRATEN DAARVAN, IN HET BIJZONDER OLODATEROL EN OLODATEROLHYDROCHLORIDE; REGISTRATION NO/DATE: RVG 112058 20131023 |
| 1224170 | SPC/GB15/018 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: NINTEDANIB*, THE TAUTOMERS AND THE SALTS THEREOF, IN PARTICULAR NINTEDANIB AND PHYSIOLOGICALLY ACCEPTABLE SALTS THEREOF, SPECIFICALLY NINTEDANIB ESILATE. *NINTEDANIB IS 3-Z-(1-(4-N-((4-METHYL-PIPERAZIN-1-YL)-METHYLCARBONYL)-N-METHYL-AMINO)-A; REGISTERED: UK EU/1/14/954/001 20141125; UK EU/1/14/954/002 20141125; UK EU/1/14/954/003 20141125; UK EU/1/14/954/004 20141125 |
| 1730131 | 2014/055 | Ireland | ⤷ Try a Trial | PRODUCT NAME: EMPAGLIFLOZIN AND SALTS THEREOF, IN PARTICULAR EMPAGLIFLOZIN ((1S)-1,5- ANHYDRO-1-C-(4-CHLORO-3-((4-(((3S)-OXOLAN-3-YL)OXY))PHENYL)METHYL)PHENYL)- D-GLUCITOL); REGISTRATION NO/DATE: EU/1/14/930 20140522 |
| 2187879 | SPC/GB17/031 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: 1-CHLORO-4-(SS-D-GLUCOPYRANOS-1-YL)-2-(4-((S)-TETRAHYDROFURAN-3-YLOXY)-BENZYL)-BENZENE (I.E. EMPAGLIFLOZIN) IN COMBINATION WITH 1-((4 METHYL-QUINAZOLIN-2-YL)METHYL)-3-METHYL-7-(2-BUTYN-1-YL)-8-(3-(R)-AMINO-PIPERIDIN-1-YL)-XANTHINE (I.E. LINAGLIPTIN) OR A P; REGISTERED: UK PLGB 14598/0191 (GB) 20161115; UK EU/1/16/1146/014(NI) 20161115; UK EU/1/16/1146/015(NI) 20161115; UK EU/1/16/1146/016(NI) 20161115; UK EU/1/16/1146/017(NI) 20161115; UK EU/1/16/1146/018(NI) 20161115; UK EU/1/16/1146/007(NI) 20161115; UK EU/1/16/1146/001(NI) 20161115; UK EU/1/16/1146/013(NI) 20161115; UK EU/1/16/1146/002(NI) 20161115; UK EU/1/16/1146/003(NI) 20161115; UK EU/1/16/1146/004(NI) 20161115; UK... |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.