BIOGEN IDEC Company Profile
✉ Email this page to a colleague
What is the competitive landscape for BIOGEN IDEC, and what generic alternatives to BIOGEN IDEC drugs are available?
BIOGEN IDEC has one approved drug.
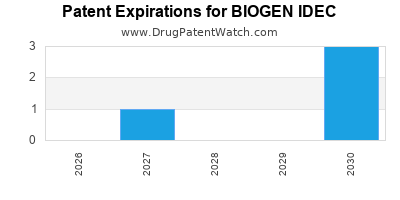
There are eight US patents protecting BIOGEN IDEC drugs.
There are eighty patent family members on BIOGEN IDEC drugs in twenty-seven countries and twenty-five supplementary protection certificates in eighteen countries.
Drugs and US Patents for BIOGEN IDEC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biogen Idec | SPINRAZA | nusinersen sodium | SOLUTION;INTRATHECAL | 209531-001 | Dec 23, 2016 | RX | Yes | Yes | 10,266,822 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Biogen Idec | SPINRAZA | nusinersen sodium | SOLUTION;INTRATHECAL | 209531-001 | Dec 23, 2016 | RX | Yes | Yes | 9,926,559 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Biogen Idec | SPINRAZA | nusinersen sodium | SOLUTION;INTRATHECAL | 209531-001 | Dec 23, 2016 | RX | Yes | Yes | 7,838,657 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Biogen Idec | SPINRAZA | nusinersen sodium | SOLUTION;INTRATHECAL | 209531-001 | Dec 23, 2016 | RX | Yes | Yes | 9,717,750 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Biogen Idec | SPINRAZA | nusinersen sodium | SOLUTION;INTRATHECAL | 209531-001 | Dec 23, 2016 | RX | Yes | Yes | 8,361,977 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BIOGEN IDEC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Biogen Idec | SPINRAZA | nusinersen sodium | SOLUTION;INTRATHECAL | 209531-001 | Dec 23, 2016 | 7,101,993 | ⤷ Try a Trial |
| Biogen Idec | SPINRAZA | nusinersen sodium | SOLUTION;INTRATHECAL | 209531-001 | Dec 23, 2016 | 6,166,197 | ⤷ Try a Trial |
| Biogen Idec | SPINRAZA | nusinersen sodium | SOLUTION;INTRATHECAL | 209531-001 | Dec 23, 2016 | 6,210,892 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for BIOGEN IDEC Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 2943225 | ⤷ Try a Trial |
| Spain | 2397113 | ⤷ Try a Trial |
| European Patent Office | 2644700 | ⤷ Try a Trial |
| South Korea | 101965868 | ⤷ Try a Trial |
| European Patent Office | 1910395 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for BIOGEN IDEC Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2548560 | 358 21-2017 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: NUSINERSEN VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/17/1188 20170601 |
| 2548560 | C02548560/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: NUSINERSEN; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 66495 20.09.2017 |
| 2548560 | 2017C/048 | Belgium | ⤷ Try a Trial | PRODUCT NAME: SPINRAZA - NUSINERSEN; AUTHORISATION NUMBER AND DATE: EU/1/17/1188 20170601 |
| 2548560 | CA 2017 00055 | Denmark | ⤷ Try a Trial | PRODUCT NAME: NUSINERSEN ELLER SALTE DERAF; REG. NO/DATE: EU/1/17/1188 20170601 |
| 2548560 | PA2017037 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: NUSINERSENAS ARBA JO DRUSKOS; REGISTRATION NO/DATE: EU/1/17/1188/001 20170530 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.