BAUSCH Company Profile
✉ Email this page to a colleague
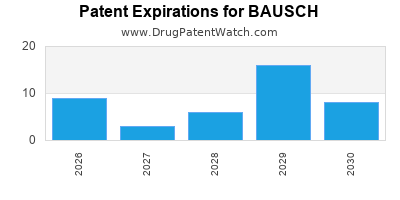
What is the competitive landscape for BAUSCH, and when can generic versions of BAUSCH drugs launch?
BAUSCH has one hundred and seventy-six approved drugs.
There are one hundred and twenty-one US patents protecting BAUSCH drugs.
There are seven hundred and seventy-one patent family members on BAUSCH drugs in fifty-two countries and two hundred and eight supplementary protection certificates in sixteen countries.
Summary for BAUSCH
| International Patents: | 771 |
| US Patents: | 121 |
| Tradenames: | 155 |
| Ingredients: | 122 |
| NDAs: | 176 |
| Patent Litigation for BAUSCH: | See patent lawsuits for BAUSCH |
Drugs and US Patents for BAUSCH
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch And Lomb | ZYLET | loteprednol etabonate; tobramycin | SUSPENSION/DROPS;OPHTHALMIC | 050804-001 | Dec 14, 2004 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bausch | CARDIZEM | diltiazem hydrochloride | TABLET;ORAL | 018602-002 | Nov 5, 1982 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bausch And Lomb | ZIRGAN | ganciclovir | GEL;OPHTHALMIC | 022211-001 | Sep 15, 2009 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BAUSCH
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch | RETIN-A MICRO | tretinoin | GEL;TOPICAL | 020475-002 | May 10, 2002 | 4,690,825 | ⤷ Try a Trial |
| Bausch | VIRAZOLE | ribavirin | FOR SOLUTION;INHALATION | 018859-001 | Dec 31, 1985 | 4,211,771 | ⤷ Try a Trial |
| Bausch | CESAMET | nabilone | CAPSULE;ORAL | 018677-001 | Dec 26, 1985 | 4,087,545 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for BAUSCH drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | For Inhalation Solution | 6 gm/vial | ➤ Subscribe | 2014-05-22 |
| ➤ Subscribe | Extended-release Tablets | 420 mg | ➤ Subscribe | 2005-04-25 |
| ➤ Subscribe | Rectal Gel | 5 mg/mL, 4mL pre-filled syringe | ➤ Subscribe | 2008-12-08 |
| ➤ Subscribe | Extended-release Tablet | 55 mg and 80 mg | ➤ Subscribe | 2010-12-02 |
| ➤ Subscribe | Gel | 1.2%/2.5% | ➤ Subscribe | 2012-12-20 |
| ➤ Subscribe | Extended-release Tablets | 150 mg and 300 mg | ➤ Subscribe | 2004-09-21 |
| ➤ Subscribe | Gel | 0.1% | ➤ Subscribe | 2010-07-08 |
| ➤ Subscribe | Ophthalmic Solution | 0.07% | ➤ Subscribe | 2013-07-26 |
| ➤ Subscribe | Capsules | 75 mg | ➤ Subscribe | 2011-06-06 |
| ➤ Subscribe | Vaginal Gel | 0.75% | ➤ Subscribe | 2004-09-02 |
| ➤ Subscribe | Cream | 5% | ➤ Subscribe | 2006-10-17 |
| ➤ Subscribe | Topical Solution | 10% | ➤ Subscribe | 2018-06-06 |
| ➤ Subscribe | Cream | 0.10% | ➤ Subscribe | 2008-01-31 |
| ➤ Subscribe | Extended-release Tablets | 348 mg | ➤ Subscribe | 2009-09-24 |
| ➤ Subscribe | Cream | 2.5% | ➤ Subscribe | 2014-06-17 |
| ➤ Subscribe | Extended-release Tablets | 120 mg, 180 mg, 240 mg, 300 mg and 360 mg | ➤ Subscribe | 2005-08-30 |
| ➤ Subscribe | Rectal Gel | 2.5 mg/0.5 mL, 5 mg/mL, 10 mg/2 mL, 15 mg/3 mL and 20 mg/4 mL | ➤ Subscribe | 2004-03-23 |
| ➤ Subscribe | Extended-release Tablets | 65 mg and 115 mg | ➤ Subscribe | 2009-11-19 |
| ➤ Subscribe | Rectal Gel | 5 mg/mL, 2mL pre-filled syringe | ➤ Subscribe | 2008-12-23 |
| ➤ Subscribe | Extended-release Tablet | 105 rng | ➤ Subscribe | 2010-12-28 |
| ➤ Subscribe | Gel | 1.2%/3.75% | ➤ Subscribe | 2015-09-30 |
| ➤ Subscribe | Ophthalmic Solution | 0.5% | ➤ Subscribe | 2012-10-19 |
| ➤ Subscribe | Gel | 0.04% | ➤ Subscribe | 2010-12-20 |
| ➤ Subscribe | Ophthalmic Solution | 1.5% | ➤ Subscribe | 2013-09-09 |
| ➤ Subscribe | Gel | 1.2%/0.025% | ➤ Subscribe | 2010-12-17 |
| ➤ Subscribe | Gel | 0.77% | ➤ Subscribe | 2006-05-10 |
| ➤ Subscribe | Lotion | 0.1% | ➤ Subscribe | 2016-08-31 |
| ➤ Subscribe | Extended-release Tablets | 174 mg | ➤ Subscribe | 2009-09-28 |
| ➤ Subscribe | Cream | 3.75% | ➤ Subscribe | 2012-08-08 |
| ➤ Subscribe | Extended-release Tablets | 522 mg | ➤ Subscribe | 2009-12-24 |
International Patents for BAUSCH Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Nicaragua | 201000015 | ⤷ Try a Trial |
| South Africa | 201301397 | ⤷ Try a Trial |
| Japan | 2018507252 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for BAUSCH Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2316456 | CA 2017 00062 | Denmark | ⤷ Try a Trial | PRODUCT NAME: NALTREXON ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, ISAER NALTREXONHYDROCHLORID, OG BUPROPION ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, ISAER BUPROPIONHYDROCHLORID; REG. NO/DATE: EU/1/14/988 20150330 |
| 3461484 | C202130024 | Spain | ⤷ Try a Trial | PRODUCT NAME: NETARSUDIL O UN ENANTIOMERO, DIASTEREIOISOMERO, SAL O SALVADO DEL MISMO EN COMBINACION CON LATANOPROST O UNA SAL DEL MISMO; NATIONAL AUTHORISATION NUMBER: EU/1/20/1502; DATE OF AUTHORISATION: 20210107; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1502; DATE OF FIRST AUTHORISATION IN EEA: 20210107 |
| 3043773 | 21C1057 | France | ⤷ Try a Trial | PRODUCT NAME: MOMETASONE OU L'UN DE SES SELS AVEC OLOPATADINE OU L'UN DE SES SELS; NAT. REGISTRATION NO/DATE: NL52121 20211026; FIRST REGISTRATION: AT - 140638 20210426 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.