ASTRAZENECA AB Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ASTRAZENECA AB, and what generic alternatives to ASTRAZENECA AB drugs are available?
ASTRAZENECA AB has twelve approved drugs.
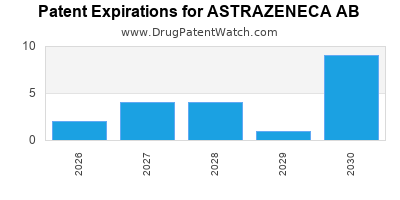
There are fifty-five US patents protecting ASTRAZENECA AB drugs. There is one tentative approval on ASTRAZENECA AB drugs.
There are eight hundred and ninety-six patent family members on ASTRAZENECA AB drugs in fifty-four countries and one hundred and forty-two supplementary protection certificates in nineteen countries.
Summary for ASTRAZENECA AB
| International Patents: | 896 |
| US Patents: | 55 |
| Tradenames: | 13 |
| Ingredients: | 10 |
| NDAs: | 12 |
| Drug Master File Entries: | 2 |
| Patent Litigation for ASTRAZENECA AB: | See patent lawsuits for ASTRAZENECA AB |
| PTAB Cases with ASTRAZENECA AB as patent owner: | See PTAB cases with ASTRAZENECA AB as patent owner |
Drugs and US Patents for ASTRAZENECA AB
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Astrazeneca Ab | BYDUREON | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-001 | Jan 27, 2012 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Astrazeneca Ab | BYDUREON | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-001 | Jan 27, 2012 | DISCN | Yes | No | 8,501,698*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | ONGLYZA | saxagliptin hydrochloride | TABLET;ORAL | 022350-001 | Jul 31, 2009 | DISCN | Yes | No | 7,951,400 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-002 | May 2, 2019 | DISCN | Yes | No | 8,716,251 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | BYDUREON | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-001 | Jan 27, 2012 | DISCN | Yes | No | 8,329,648*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | XIGDUO XR | dapagliflozin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 205649-005 | Jul 28, 2017 | RX | Yes | No | 7,919,598 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ASTRAZENECA AB
Paragraph IV (Patent) Challenges for ASTRAZENECA AB drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 250 mg/mL, 1.2 mL and 2.4 mL prefilled syringe | ➤ Subscribe | 2014-06-11 |
| ➤ Subscribe | HydrochlorideExtended-release Tablets | 2.5 mg/1000 mg | ➤ Subscribe | 2018-10-29 |
| ➤ Subscribe | Extended-release Tablets | 5 mg/500 mg, 2.5 mg/1000 mg, and 5 mg/1000 mg | ➤ Subscribe | 2013-07-31 |
| ➤ Subscribe | Tablets | 2.5 mg and 5 mg | ➤ Subscribe | 2013-07-31 |
International Patents for ASTRAZENECA AB Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Finland | 2508188 | ⤷ Try a Trial |
| South Korea | 20120064141 | ⤷ Try a Trial |
| Portugal | 2992098 | ⤷ Try a Trial |
| Egypt | 24515 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 03099836 | ⤷ Try a Trial |
| European Patent Office | 2298288 | ⤷ Try a Trial |
| Slovenia | 2992098 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for ASTRAZENECA AB Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1261586 | 12C0028 | France | ⤷ Try a Trial | PRODUCT NAME: ASSOCIATION COMPRENANT LA SAXAGLIPTINE OU UN DE SES SELS ET LA METFORMINE OU UN DE SES SELS, Y COMPRIS L'ASSOCIATION CHLORHYDRATE DE SAXAGLIPTINE ET CHLORHYDRATE DE METFORMINE; REGISTRATION NO/DATE: EU/1/11/731/001 20111124 |
| 1506211 | 136 5005-2013 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOZIN; FIRST REGISTRATION NO/DATE: EU/1/12/795/001, EU/1/12/795/002, EU/1/12/795/003, EU/1/12/795/004, EU/1/12/795/005, EU/1/12/795/006, EU/1/12/795/007, EU/1/12/795/007, EU/1/12/795/008, EU/1/12/795/009, EU/1/12/795/010 20121112 |
| 1506211 | 92182 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOZINE ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES |
| 2435025 | LUC00124 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: UNE COMBINAISON DE GLYCOPYRROLATE (Y COMPRIS SES SELS, ESTERS, ENANTIOMERES OU AUTRES DERIVES PHARMACEUTIQUEMENT ACCEPTABLES) ET DE FORMOTEROL (Y COMPRIS SES SELS, ESTERS, ENANTIOMERES OU AUTRES DERIVES PHARMACEUTIQUEMENT ACCEPTABLES); AUTHORISATION NUMBER AND DATE: EU/1/18/1339 20181220 |
| 1532149 | CA 2013 00001 | Denmark | ⤷ Try a Trial | PRODUCT NAME: 8-(3-AMINOPIPERIDIN-1-YL)-7-BUT-2-INYL-3-METHYL-1-(4-METHYLCHINAZOLIN-2-YLMETHYL)-3,7-DIHYDROPURIN-2,6-DION ENANTIOMERER OG SALTE DERAF - SAERLIGT LINAGLIPTIN - I KOMBINATION MED METFORMINHYDROCHLORID; REG. NO/DATE: EU/1/12/780/001-028 20120720 |
| 2139494 | CA 2020 00035 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTIN OG DAPAGLIFLOZIN; REG. NO/DATE: EU/1/16/1108 20160719 |
| 2435024 | LUC00208 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE FORMOTEROL (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES), DE GLYCOPYRRONIUM (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES) ET DE BUDESONIDE (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES); AUTHORISATION NUMBER AND DATE: EU/1/20/1468 20201210 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.