AMNEAL Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AMNEAL, and when can generic versions of AMNEAL drugs launch?
AMNEAL has three hundred and eighty-four approved drugs.
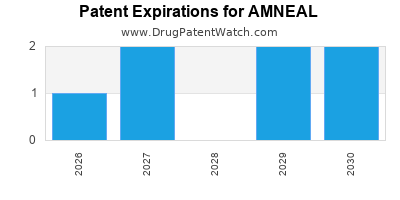
There are five US patents protecting AMNEAL drugs. There are seven tentative approvals on AMNEAL drugs.
There are fifteen patent family members on AMNEAL drugs in twelve countries and seven hundred and ninety-nine supplementary protection certificates in eighteen countries.
Summary for AMNEAL
| International Patents: | 15 |
| US Patents: | 5 |
| Tradenames: | 291 |
| Ingredients: | 283 |
| NDAs: | 384 |
| Patent Litigation for AMNEAL: | See patent lawsuits for AMNEAL |
| PTAB Cases with AMNEAL as petitioner: | See PTAB cases with AMNEAL as petitioner |
Drugs and US Patents for AMNEAL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amneal Pharms Co | DOXYCYCLINE HYCLATE | doxycycline hyclate | TABLET;ORAL | 209372-001 | Oct 6, 2017 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Amneal Pharms | TEMOZOLOMIDE | temozolomide | CAPSULE;ORAL | 203691-006 | May 8, 2015 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal | ETRAVIRINE | etravirine | TABLET;ORAL | 214196-003 | Jun 14, 2021 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal Pharms Ny | ESOMEPRAZOLE MAGNESIUM | esomeprazole magnesium | CAPSULE, DELAYED REL PELLETS;ORAL | 209647-002 | Apr 10, 2019 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Amneal | PIRFENIDONE | pirfenidone | TABLET;ORAL | 212570-002 | Mar 25, 2022 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal | VANCOMYCIN HYDROCHLORIDE | vancomycin hydrochloride | FOR SOLUTION;ORAL | 215338-001 | Jun 23, 2023 | AA | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amneal Pharms | VIGABATRIN | vigabatrin | FOR SOLUTION;ORAL | 210155-001 | Mar 13, 2018 | AA | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for AMNEAL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 5,466,699*PED | ⤷ Try a Trial |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 6,750,237*PED | ⤷ Try a Trial |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-003 | Sep 16, 2013 | 6,750,237*PED | ⤷ Try a Trial |
| Amneal | ACTIVELLA | estradiol; norethindrone acetate | TABLET;ORAL | 020907-001 | Nov 18, 1998 | RE36247 | ⤷ Try a Trial |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-004 | Sep 30, 2003 | 7,220,767*PED | ⤷ Try a Trial |
| Amneal | ZOMIG | zolmitriptan | SPRAY;NASAL | 021450-003 | Sep 16, 2013 | 7,220,767*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for AMNEAL drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Nasal Spray | 5 mg/spray | ➤ Subscribe | 2013-11-14 |
| ➤ Subscribe | for Injection | 100 mcg/vial and 500 mcg/vial | ➤ Subscribe | 2015-04-14 |
| ➤ Subscribe | Injection | 100 mg/mL, 2.5 mL vials | ➤ Subscribe | 2007-09-24 |
| ➤ Subscribe | Nasal Spray | 2.5 mg/spray | ➤ Subscribe | 2016-06-09 |
| ➤ Subscribe | for Injection | 200 mcg/vial | ➤ Subscribe | 2015-05-01 |
| ➤ Subscribe | Injection | 1 mg/mL, 50 mL vials | ➤ Subscribe | 2011-12-16 |
International Patents for AMNEAL Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 114450002 | ⤷ Try a Trial |
| Portugal | 2881109 | ⤷ Try a Trial |
| Canada | 3148812 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2021021277 | ⤷ Try a Trial |
| European Patent Office | 4003323 | ⤷ Try a Trial |
| Spain | 2795421 | ⤷ Try a Trial |
| Poland | 2881109 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for AMNEAL Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2673237 | LUC00111 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SODIUM ZIRCONIUM CYCLOSILICATE; AUTHORISATION NUMBER AND DATE: EU/1/17/1173 20180326 |
| 0934061 | PA2004017 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: PREGABALINUM ((S)-3-(AMINOMETIL)-5-METILHEKSANO RûGðTIS) |
| 0810209 | SPC/GB07/038 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: DARUNAVIR OR THE PHARMACEUTICALLY ACCEPTABLE SALT, ESTER OR PRODRUG THEREOF; REGISTERED: UK EU/1/06/380/001 20070212; SPC EXTENSION AUTHORISATION: EU/1/06/380/001 - 008, 20070212 |
| 2962690 | C201930044 | Spain | ⤷ Try a Trial | PRODUCT NAME: APREMILAST O UNA SAL FARMACEUTICAMENTE ACEPTABLE DEL MISMO; NATIONAL AUTHORISATION NUMBER: EU/1/14/981; DATE OF AUTHORISATION: 20150115; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/14/981; DATE OF FIRST AUTHORISATION IN EEA: 20150115 |
| 2782584 | 132021000000197 | Italy | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOLO (17SS-ESTRADIOLO) IN PARTICOLARE NELLA FORMA EMIIDRATA, E PROGESTERONE COMPRENDENTI LE VARIE FORME DI ESTRADIOLO (17SS-ESTRADIOLO), QUALI LE FORME IDRATE E SOLVATATE, INCLUDENDO LA FORMA EMIIDRATA, ED I SUOI SALI.(BIJUVA); AUTHORISATION NUMBER(S) AND DATE(S): BE582231, 20210406;048335018 -048335020, 20210517 |
| 0633893 | SPC/GB11/063 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ABIRATERONE AND ACID ADDITION SALTS AND 3-ESTERS THEREOF ESPECIALLY ABIRATERONE ACETATE; REGISTERED: UK EU/1/11/714/001 20110905 |
| 3412676 | PA2020519,C3412676 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: VABORBAKTAMO IR (ARBA) JO DRUSKOS, IR (ARBA) HIDRATO BEI MEROPENEMO IR (ARBA) JO DRUSKOS, IR (ARBA) HIDRATO, YPAC MEROPENEMO TRIHIDRATO, DERINYS; REGISTRATION NO/DATE: EU/1/18/1334 20181120 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.