AM REGENT Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AM REGENT, and when can generic versions of AM REGENT drugs launch?
AM REGENT has seventy-seven approved drugs.
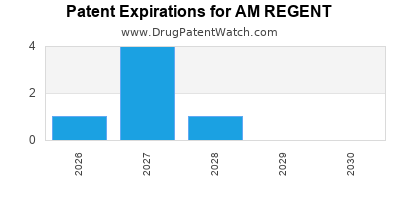
There are seven US patents protecting AM REGENT drugs.
There are sixty-seven patent family members on AM REGENT drugs in thirty-two countries and one hundred and forty-three supplementary protection certificates in fifteen countries.
Drugs and US Patents for AM REGENT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Am Regent | ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE | ondansetron hydrochloride | INJECTABLE;INJECTION | 079032-001 | Nov 18, 2008 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | CAFFEINE CITRATE | caffeine citrate | SOLUTION;INTRAVENOUS | 077906-001 | May 15, 2007 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Am Regent | GANCICLOVIR SODIUM | ganciclovir sodium | INJECTABLE;INJECTION | 202624-001 | Sep 18, 2013 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | FLOXURIDINE | floxuridine | INJECTABLE;INJECTION | 203008-001 | Nov 22, 2017 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | ZINC SULFATE | zinc sulfate | SOLUTION;INTRAVENOUS | 209377-002 | Jul 18, 2019 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-002 | Oct 8, 2020 | RX | Yes | Yes | 7,754,702 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for AM REGENT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-002 | Oct 8, 2020 | 10,519,252 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-003 | Apr 28, 2021 | 11,291,645 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-002 | Oct 8, 2020 | 11,590,097 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-003 | Apr 28, 2021 | 11,590,097 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-004 | Feb 4, 2022 | 11,123,321 | ⤷ Try a Trial |
| Am Regent | DEXFERRUM | ferric oxyhydroxide | INJECTABLE;INJECTION | 040024-001 | Feb 23, 1996 | 5,624,668 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for AM REGENT Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Norway | 333008 | ⤷ Try a Trial |
| Germany | 50313653 | ⤷ Try a Trial |
| China | 116096423 | ⤷ Try a Trial |
| Japan | 5289413 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2007081744 | ⤷ Try a Trial |
| Slovenia | 2287204 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for AM REGENT Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0720599 | CR 2014 00050 | Denmark | ⤷ Try a Trial | PRODUCT NAME: EZETIMIBE AND ATORVASTATIN OR PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, INCLUDING ATORVASTATIN AS ATORVASTATIN CALCIUM TRIHYDRATE; REG. NO/DATE: DE/H/3895-3898/001-004/DC 20140910 |
| 3141251 | 301099 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: A MEDICINAL PRODUCT CONSISTING OF A COMBINATION OF A FIRST DOSE PHARMACEUTICAL COMPOSITION AND A SECOND DOSE PHARMACEUTICAL COMPOSITION, THE FIRST DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, SODIUM SULPHATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE AND THE SECOND DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, ASCORBIC ACID, SODIUM ASCORBATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE; NATIONAL REGISTRATION NO/DATE: RVG 120195 20171114; FIRST REGISTRATION: IS IS/1/17/083/01 20171016 |
| 2957286 | 300962 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: PATIROMER SORBITEX CALCIUM; REGISTRATION NO/DATE: EU/1/17/1179 20170721 |
| 1853250 | 300673 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: PACLITAXEL, GEFORMULEERD ALS ALBUMINE-GEBONDEN NANODEELTJES; REGISTRATION NO/DATE: EU/1/07/428/001-002 20131220 |
| 2957286 | 132019000000021 | Italy | ⤷ Try a Trial | PRODUCT NAME: PATIROMER SORBITEX CALCIUM E QUALSIASI SUO SALE O DERIVATO(VELTASSA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/17/1179, 20170721 |
| 0502314 | SPC/GB02/037 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TELMISARTAN, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, AND HYDROCHLOROTHIAZIDE; REGISTERED: UK EU/1/02/213/001 20020419; UK EU/1/02/213/002 20020419; UK EU/1/02/213/003 20020419; UK EU/1/02/214/004 20020419; UK EU/1/02/213/005 20020419; UK EU/1/02/213/006 20020419; UK EU/1/02/213/007 20020419; UK EU/1/02/213/008 20020419; UK EU/1/02/213/009 20020419; UK EU/1/02/213/010 20020419 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.