JAKAFI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Jakafi, and what generic alternatives are available?
Jakafi is a drug marketed by Incyte Corp and is included in one NDA. There are eight patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and thirty-three patent family members in forty-six countries.
The generic ingredient in JAKAFI is ruxolitinib phosphate. There are two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the ruxolitinib phosphate profile page.
DrugPatentWatch® Generic Entry Outlook for Jakafi
Jakafi was eligible for patent challenges on November 16, 2015.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 12, 2028. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for JAKAFI
| International Patents: | 233 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 65 |
| Clinical Trials: | 75 |
| Patent Applications: | 514 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for JAKAFI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for JAKAFI |
| What excipients (inactive ingredients) are in JAKAFI? | JAKAFI excipients list |
| DailyMed Link: | JAKAFI at DailyMed |
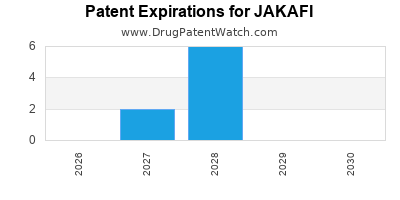

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for JAKAFI
Generic Entry Date for JAKAFI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for JAKAFI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Medical College of Wisconsin | Phase 2 |
| Ohio State University Comprehensive Cancer Center | Phase 2 |
| Telios Pharma, Inc. | Phase 1/Phase 2 |
Pharmacology for JAKAFI
| Drug Class | Janus Kinase Inhibitor Kinase Inhibitor |
| Mechanism of Action | Janus Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for JAKAFI
Paragraph IV (Patent) Challenges for JAKAFI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| JAKAFI | Tablets | ruxolitinib phosphate | 5 mg, 10 mg, 15 mg, 20 mg, and 25 mg | 202192 | 1 | 2015-12-17 |
US Patents and Regulatory Information for JAKAFI
JAKAFI is protected by eight US patents and eight FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of JAKAFI is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting JAKAFI
Salts of the janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-D]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentyl- propanenitrile
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pyrazolyl substituted pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Salts of the Janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentyl- propanenitrile
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Salts of the janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-d] pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Salts of the Janus kinase inhibitor (R)-3-(4-(7H-pyrrolo[2,3-D]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentyl- propanenitrile
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as Janus kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Heteroaryl substituted pyrrolo[2,3-B] pyridines and pyrrolo[2,3-B] pyrimidines as janus kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting JAKAFI
TREATMENT OF STEROID-REFRACTORY ACUTE GRAFT-VERSUS-HOST DISEASE (GVHD) IN ADULT AND PEDIATRIC PATIENTS 12 YEARS AND OLDER
Exclusivity Expiration: ⤷ Try a Trial
ADDITION OF THE INDICATION OF TREATMENT OF CHRONIC GRAFT-VERSUS-HOST DISEASE (CGVHD) AFTER FAILURE OF ONE OR TWO LINES OF SYSTEMIC THERAPY IN ADULT AND PEDIATRIC PATIENTS 12 YEARS AND OLDER
Exclusivity Expiration: ⤷ Try a Trial
REVISIONS TO SECTION 8.4 OF THE LABELING TO INCLUDE THE RESULTS OF STUDY INCB 18424-269
Exclusivity Expiration: ⤷ Try a Trial
TREATMENT OF CHRONIC GRAFT-VERSUS-HOST DISEASE (CGVHD) AFTER FAILURE OF ONE OR TWO LINES OF SYSTEMIC THERAPY IN ADULT AND PEDIATRIC PATIENTS 12 YEARS AND OLDER
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-005 | Nov 16, 2011 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-003 | Nov 16, 2011 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-002 | Nov 16, 2011 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-004 | Nov 16, 2011 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-004 | Nov 16, 2011 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for JAKAFI
When does loss-of-exclusivity occur for JAKAFI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 08266183
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0814254
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 89663
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1932582
Estimated Expiration: ⤷ Try a Trial
Patent: 3524509
Estimated Expiration: ⤷ Try a Trial
Colombia
Patent: 51256
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 151
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0140541
Estimated Expiration: ⤷ Try a Trial
Patent: 0160717
Estimated Expiration: ⤷ Try a Trial
Patent: 0190385
Estimated Expiration: ⤷ Try a Trial
Cuba
Patent: 933
Estimated Expiration: ⤷ Try a Trial
Patent: 179
Estimated Expiration: ⤷ Try a Trial
Patent: 090213
Estimated Expiration: ⤷ Try a Trial
Patent: 120155
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 15145
Estimated Expiration: ⤷ Try a Trial
Patent: 17693
Estimated Expiration: ⤷ Try a Trial
Patent: 21338
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 73752
Estimated Expiration: ⤷ Try a Trial
Patent: 40731
Estimated Expiration: ⤷ Try a Trial
Patent: 70090
Estimated Expiration: ⤷ Try a Trial
Dominican Republic
Patent: 009000280
Estimated Expiration: ⤷ Try a Trial
Ecuador
Patent: 099802
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 9784
Estimated Expiration: ⤷ Try a Trial
Patent: 1070013
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 73752
Estimated Expiration: ⤷ Try a Trial
Patent: 40731
Estimated Expiration: ⤷ Try a Trial
Patent: 70090
Estimated Expiration: ⤷ Try a Trial
Patent: 95369
Estimated Expiration: ⤷ Try a Trial
Patent: 11883
Estimated Expiration: ⤷ Try a Trial
Georgia, Republic of
Patent: 0125533
Estimated Expiration: ⤷ Try a Trial
Guatemala
Patent: 0900314
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 43161
Estimated Expiration: ⤷ Try a Trial
Patent: 98652
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 29236
Estimated Expiration: ⤷ Try a Trial
Patent: 43732
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 2524
Estimated Expiration: ⤷ Try a Trial
Patent: 4276
Estimated Expiration: ⤷ Try a Trial
Patent: 0401
Estimated Expiration: ⤷ Try a Trial
Patent: 7708
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 75653
Estimated Expiration: ⤷ Try a Trial
Patent: 10529209
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 70090
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 4969
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 2814
Estimated Expiration: ⤷ Try a Trial
Patent: 09013402
Estimated Expiration: ⤷ Try a Trial
Montenegro
Patent: 960
Estimated Expiration: ⤷ Try a Trial
Morocco
Patent: 517
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 1803
Estimated Expiration: ⤷ Try a Trial
Nicaragua
Patent: 0900216
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 19025
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 73752
Estimated Expiration: ⤷ Try a Trial
Patent: 40731
Estimated Expiration: ⤷ Try a Trial
Patent: 70090
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 73752
Estimated Expiration: ⤷ Try a Trial
Patent: 70090
Estimated Expiration: ⤷ Try a Trial
San Marino
Patent: 201000002
Estimated Expiration: ⤷ Try a Trial
Patent: 01000002
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 245
Estimated Expiration: ⤷ Try a Trial
Patent: 878
Estimated Expiration: ⤷ Try a Trial
Patent: 449
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 2198
Estimated Expiration: ⤷ Try a Trial
Patent: 201509887U
Estimated Expiration: ⤷ Try a Trial
Patent: 201912675V
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 73752
Estimated Expiration: ⤷ Try a Trial
Patent: 40731
Estimated Expiration: ⤷ Try a Trial
Patent: 70090
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0908826
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1549876
Estimated Expiration: ⤷ Try a Trial
Patent: 100049010
Estimated Expiration: ⤷ Try a Trial
Patent: 150036210
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 67665
Estimated Expiration: ⤷ Try a Trial
Patent: 75797
Estimated Expiration: ⤷ Try a Trial
Patent: 14092
Estimated Expiration: ⤷ Try a Trial
Patent: 03444
Estimated Expiration: ⤷ Try a Trial
Tunisia
Patent: 09000514
Estimated Expiration: ⤷ Try a Trial
Turkey
Patent: 1903488
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 467
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering JAKAFI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Portugal | 2343299 | ⤷ Try a Trial | |
| Taiwan | I410407 | ⤷ Try a Trial | |
| Georgia, Republic of | P20125533 | SALTS OF THE JANUS KINASE INHIBITOR (R)-3-(4-(7H-PYRROLO[2,3-D]PYRIMIDIN-4-YL)-1H-PYRAZOL-1-YL)-3-CYCLOPENTYL PROPANENITRILE | ⤷ Try a Trial |
| Croatia | P20160112 | ⤷ Try a Trial | |
| New Zealand | 778831 | Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for JAKAFI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1966202 | SPC/GB13/005 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: RUXOLITINIB, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF.; REGISTERED: UK EU/1/12/773/001 20120828; UK EU/1/12/773/002 20120828; UK EU/1/12/773/003 20120828 |
| 2455382 | 122017000020 | Germany | ⤷ Try a Trial | PRODUCT NAME: RUXOLITINIB, ODER EIN PHARMAZEUTISCH AKZEPTABLES SALZ DAVON; REGISTRATION NO/DATE: EU/1/12/773/001-003 20150311 |
| 1966202 | C01966202/01 | Switzerland | ⤷ Try a Trial | FORMER OWNER: INCYTE CORPORATION, US |
| 1966202 | 1390005-5 | Sweden | ⤷ Try a Trial | PRODUCT NAME: RUXOLITINIB, ELLER ETT FARMACEUTISKT GODTAGBART SALT DAERAV; REG. NO/DATE: EU/1/12/773/001; 2012-08-23; PERIOD OF VALIDITY (FROM - UNTIL): 2026-12-13 - 2027-08-27 |
| 1966202 | C20130003 00072 | Estonia | ⤷ Try a Trial | PRODUCT NAME: RUKSOLITINIIB;REG NO/DATE: K(2012)6018 LOPLIK 23.08.2012 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


