XIFAXAN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Xifaxan, and what generic alternatives are available?
Xifaxan is a drug marketed by Salix Pharms and is included in one NDA. There are twenty-nine patents protecting this drug and two Paragraph IV challenges.
This drug has two hundred and eight patent family members in forty countries.
The generic ingredient in XIFAXAN is rifaximin. There are fourteen drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the rifaximin profile page.
DrugPatentWatch® Generic Entry Outlook for Xifaxan
Xifaxan was eligible for patent challenges on May 25, 2008.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 2, 2027. This may change due to patent challenges or generic licensing.
There have been six patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are two tentative approvals for the generic drug (rifaximin), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for XIFAXAN
| International Patents: | 208 |
| US Patents: | 29 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 55 |
| Clinical Trials: | 39 |
| Patent Applications: | 5,218 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for XIFAXAN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for XIFAXAN |
| What excipients (inactive ingredients) are in XIFAXAN? | XIFAXAN excipients list |
| DailyMed Link: | XIFAXAN at DailyMed |
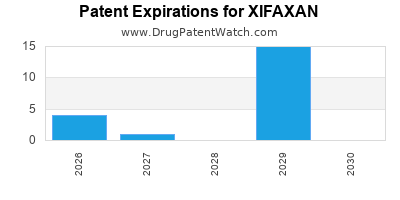
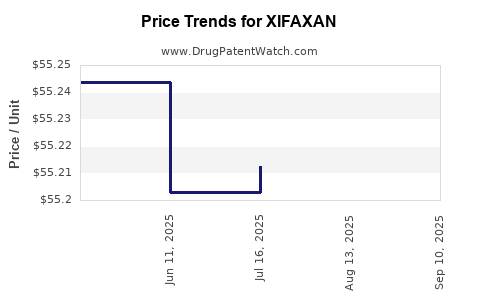
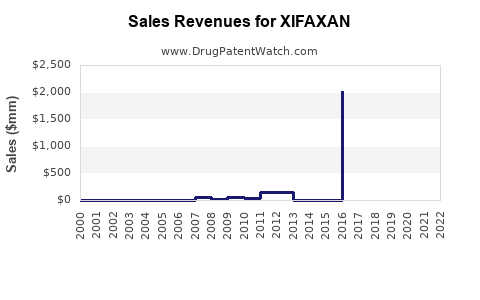
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for XIFAXAN
Generic Entry Dates for XIFAXAN*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
Generic Entry Dates for XIFAXAN*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for XIFAXAN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Ronnie Fass, MD | Phase 3 |
| MetroHealth Medical Center | Phase 3 |
| Cedars-Sinai Medical Center | Phase 1/Phase 2 |
Pharmacology for XIFAXAN
| Drug Class | Rifamycin Antibacterial |
Anatomical Therapeutic Chemical (ATC) Classes for XIFAXAN
US Patents and Regulatory Information for XIFAXAN
XIFAXAN is protected by forty US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of XIFAXAN is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting XIFAXAN
Methods of treating hepatic encephalopathy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION IN RISK OF OVERT HEPATIC ENCEPHALOPATHY (HE) RECURRENCE
Methods of treating hepatic encephalopathy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION IN A SUBJECT'S RISK OF EXPERIENCING A BREAKTHROUGH OVERT HEPATIC ENCEPHALOPATHY (HE) EPISODE
Methods for treating irritable bowel syndrome (IBS)
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS 65 YEARS OF AGE OR OLDER AND SYMPTOMS THEREOF
Methods for treating irritable bowel syndrome (IBS)
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS 65 YEARS OF AGE OR OLDER
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS.
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF TRAVELERS' DIARRHEA (TD) CAUSED BY NONINVASIVE STRAINS OF ESCHERIA COLI IN ADULT AND PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION IN RISK OF OVERT HEPATIC ENCEPHALOPATHY (HE) RECURRENCE IN ADULTS
Methods of treating hepatic encephalopathy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION IN A SUBJECT'S RISK OF EXPERIENCING A BREAKTHROUGH OVERT HEPATIC ENCEPHALOPATHY (HE) EPISODE
Methods for treating irritable bowel syndrome (IBS)
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS 65 YEARS OF AGE OR OLDER
Methods for treating irritable bowel syndrome (IBS)
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS 65 YEARS OF AGE OR OLDER AND SYMPTOMS THEREOF
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULT FEMALE SUBJECTS AND SYMPTOMS THEREOF
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULT FEMALE SUBJECTS
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF BLOATING ASSOCIATED WITH DIARRHEA-PREDOMINANT IRRITABLE BOWEL SYNDROME (IBS-D) IN ADULT FEMALE SUBJECTS
Polymorphous forms of rifaximin, processes for their production and use thereof in medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Polymorphic forms .alpha., .beta., and .gamma. of rifaximin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Polymorphic forms .alpha., .beta. and .gamma. of rifaximin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical compositions comprising polymorphic forms .alpha., .beta., and .gamma. of rifaximin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Use of polymorphic forms of rifaximin for medical preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS.
Use of polymorphic forms of rifaximin for medical preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS AND SYMPTOMS THEREOF.
Methods of treating travelers diarrhea and hepatic encephalopathy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING TRAVELERS' DIARRHEA
Pharmaceutical compositions comprising polymorphic forms .alpha., .beta., and .gamma. of rifaximin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Polymorphic forms .alpha., .beta. and .gamma. of rifaximin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS AND SYMPTOMS THEREOF.
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS.
Methods for treating diarrhea-associated irritable bowel syndrome
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS.
Methods for treating diarrhea-associated irritable bowel syndrome
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS AND SYMPTOMS THEREOF.
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods of treating hepatic encephalopathy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION IN RISK OF OVERT HEPATIC ENCEPHALOPATHY (HE) RECURRENCE
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF PATIENTS WITH TRAVELERS' DIARRHEA (TD) OR THE REDUCTION IN RISK OF OVERT HEPATIC ENCEPHALOPATHY (HE) RECURRENCE
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS.
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF IRRITABLE BOWEL SYNDROME WITH DIARRHEA (IBS-D) IN ADULTS AND SYMPTOMS THEREOF.
Methods of treating traveler's diarrhea and hepatic encephalopathy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF PATIENTS WITH HEPATIC ENCEPHALOPATHY (HE)
Polymorphic forms .alpha., .beta. and .gamma. of rifaximin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical compositions comprising polymorphic forms .alpha., .beta., and .gamma. of rifaximin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods of treating traveler's diarrhea and hepatic encephalopathy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION IN RISK OF OVERT HEPATIC ENCEPHALOPATHY (HE) RECURRENCE
Methods of treating hepatic encephalopathy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION IN RISK OF OVERT HEPATIC ENCEPHALOPATHY (HE) RECURRENCE
Polymorphous forms of rifaximin, processes for their production and use thereof in the medicinal preparations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods of treating hepatic encephalopathy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION IN RISK OF OVERT HEPATIC ENCEPHALOPATHY (HE) RECURRENCE
Methods of treating traveler's diarrhea and hepatic encephalopathy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION IN RISK OF OVERT HEPATIC ENCEPHALOPATHY (HE) IN ADULTS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salix Pharms | XIFAXAN | rifaximin | TABLET;ORAL | 021361-002 | Mar 24, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Salix Pharms | XIFAXAN | rifaximin | TABLET;ORAL | 021361-002 | Mar 24, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Salix Pharms | XIFAXAN | rifaximin | TABLET;ORAL | 021361-002 | Mar 24, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Salix Pharms | XIFAXAN | rifaximin | TABLET;ORAL | 021361-002 | Mar 24, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for XIFAXAN
When does loss-of-exclusivity occur for XIFAXAN?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 06222312
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0608073
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 94789
Estimated Expiration: ⤷ Try a Trial
China
Patent: 00077
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0070433
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 98630
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 98630
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 96396
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 37825
Estimated Expiration: ⤷ Try a Trial
Patent: 08531623
Estimated Expiration: ⤷ Try a Trial
Patent: 13216710
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 07010742
Estimated Expiration: ⤷ Try a Trial
Moldova, Republic of
Patent: 81
Estimated Expiration: ⤷ Try a Trial
Patent: 070265
Estimated Expiration: ⤷ Try a Trial
Montenegro
Patent: 272
Estimated Expiration: ⤷ Try a Trial
Patent: 4408
Estimated Expiration: ⤷ Try a Trial
Morocco
Patent: 345
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 6737
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 2197
Estimated Expiration: ⤷ Try a Trial
Patent: 074988
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 98630
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 98630
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 97985
Estimated Expiration: ⤷ Try a Trial
Patent: 07136430
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 570
Estimated Expiration: ⤷ Try a Trial
Patent: 070364
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 98630
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0707397
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 0885755
Estimated Expiration: ⤷ Try a Trial
Patent: 060096242
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 22895
Estimated Expiration: ⤷ Try a Trial
Tunisia
Patent: 07292
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 809
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering XIFAXAN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Germany | 602004019298 | ⤷ Try a Trial | |
| Austria | 361927 | ⤷ Try a Trial | |
| Brazil | PI1010028 | métodos de tratamento de encefalopatia hepática | ⤷ Try a Trial |
| Mexico | 2011012829 | METODOS PARA TRATAR ENCEFALOPATIA HEPATICA. (METHODS OF TREATING HEPATIC ENCEPHALOPATHY.) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


