VICTRELIS Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Victrelis, and when can generic versions of Victrelis launch?
Victrelis is a drug marketed by Merck Sharp Dohme and is included in one NDA. There are three patents protecting this drug.
This drug has one hundred and ten patent family members in thirty-three countries.
The generic ingredient in VICTRELIS is boceprevir. Additional details are available on the boceprevir profile page.
DrugPatentWatch® Generic Entry Outlook for Victrelis
Victrelis was eligible for patent challenges on May 13, 2015.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 11, 2027. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for VICTRELIS
| International Patents: | 110 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 54 |
| Clinical Trials: | 9 |
| Patent Applications: | 1,634 |
| Formulation / Manufacturing: | see details |
| DailyMed Link: | VICTRELIS at DailyMed |
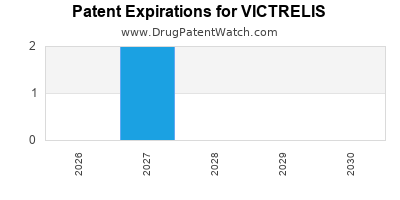
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VICTRELIS
Generic Entry Date for VICTRELIS*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VICTRELIS
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Hoffmann-La Roche | |
| UMC Utrecht | Phase 2 |
| Radboud University | Phase 2 |
Anatomical Therapeutic Chemical (ATC) Classes for VICTRELIS
US Patents and Regulatory Information for VICTRELIS
VICTRELIS is protected by three US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VICTRELIS is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting VICTRELIS
Pharmaceutical formulations and methods of treatment using the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF CHRONIC HEPATITIS C (CHC) GENOTYPE 1 INFECTION IN COMBINATION WITH PEGINTERFERON ALFA AND RIBAVIRIN IN ADULT PATIENTS (>=18 YEARS OF AGE) WITH COMPENSATED LIVER DISEASE
Administration of HCV protease inhibitors in combination with food to improve bioavailability
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF CHRONIC HEPATITIS C (CHC) GENOTYPE 1 INFECTION, ADMINISTERED WITH FOOD
Peptides as NS3-serine protease inhibitors of hepatitis C virus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF CHRONIC HEPATITIS C (CHC) GENOTYPE 1 INFECTION IN COMBINATION WITH PEGINTERFERON ALFA AND RIBAVIRIN IN ADULT PATIENTS (>=18 YEARS OF AGE) WITH COMPENSATED LIVER DISEASE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Merck Sharp Dohme | VICTRELIS | boceprevir | CAPSULE;ORAL | 202258-001 | May 13, 2011 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Merck Sharp Dohme | VICTRELIS | boceprevir | CAPSULE;ORAL | 202258-001 | May 13, 2011 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Merck Sharp Dohme | VICTRELIS | boceprevir | CAPSULE;ORAL | 202258-001 | May 13, 2011 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for VICTRELIS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Merck Sharp Dohme | VICTRELIS | boceprevir | CAPSULE;ORAL | 202258-001 | May 13, 2011 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for VICTRELIS
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Merck Sharp Dohme Ltd | Victrelis | boceprevir | EMEA/H/C/002332 Victrelis is indicated for the treatment of chronic hepatitis-C (CHC) genotype-1 infection, in combination with peginterferon alfa and ribavirin, in adult patients with compensated liver disease who are previously untreated or who have failed previous therapy. |
Withdrawn | no | no | no | 2011-07-18 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for VICTRELIS
When does loss-of-exclusivity occur for VICTRELIS?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5198
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 06252553
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0610737
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 11155
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1212970
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 19478
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 60415
Estimated Expiration: ⤷ Try a Trial
Patent: 08542383
Estimated Expiration: ⤷ Try a Trial
Patent: 12250996
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 07015270
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 3365
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 076613
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 070011
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0709987
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 080021634
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 72980
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 28138
Estimated Expiration: ⤷ Try a Trial
Patent: 0716157
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VICTRELIS around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| World Intellectual Property Organization (WIPO) | 2006130628 | ⤷ Try a Trial | |
| Brazil | 0306931 | ⤷ Try a Trial | |
| Luxembourg | 91910 | ⤷ Try a Trial | |
| Japan | 2012250996 | PHARMACEUTICAL FORMULATION AND METHOD OF TREATMENT USING THE SAME | ⤷ Try a Trial |
| South Korea | 20080021634 | PHARMACEUTICAL FORMULATIONS AND METHODS OF TREATMENT USING THE SAME | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VICTRELIS
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1385870 | 300506 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: BOCEPREVIR, OF EEN ENANTIOMEER, STEREOISIOMEER, ROTAMEER, TAUTOMEER OF RACEMAAT VAN DIE VERBINDING, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF SOLVAAT; REGISTRATION NO/DATE: EU/1/11/704/001 20110718 |
| 1385870 | 12C0002 | France | ⤷ Try a Trial | PRODUCT NAME: BOCEPREVIR OU UN ENANTIOMERE, STEREOISOMERE, ROTAMERE, TAUTOMERE OU RACEMATE DUDIT COMPOSE, OU UN DE SES SELS OU SOLVATES PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/11/704/001 20110718 |
| 1385870 | C300506 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: BOCEPREVIR, OF EEN ENANTIOMEER, STEREOISIOMEER, ROATMEER, TAUTOMEER OF RACEMAAT VAN DIE VERBINDING, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF SOLVAAT; REGISTRATION NO/DATE: EU/1/11/704/001 20110718 |
| 1385870 | CA 2011 00036 | Denmark | ⤷ Try a Trial | PRODUCT NAME: BOCEPREVIR ELLER EN ENANTIOMER, EN STEREOISOMER, EN ROTAMER, EN TAUTOMER ELLER ET RACEMAT AF DENNE FORBINDELSE, ELLER FARMACEUTISK ACCEPTABELT SALT ELLER SOLVAT AF DENNE FORBINDEL |
| 1385870 | SPC/GB11/057 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: BOCEPREVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/11/704/001 20110720 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


