RECLAST Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Reclast, and when can generic versions of Reclast launch?
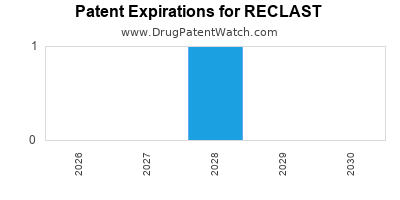
Reclast is a drug marketed by Sandoz and is included in one NDA. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has twenty-six patent family members in twenty-two countries.
The generic ingredient in RECLAST is zoledronic acid. There are twenty-five drug master file entries for this compound. Twenty-three suppliers are listed for this compound. Additional details are available on the zoledronic acid profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Reclast
A generic version of RECLAST was approved as zoledronic acid by DR REDDYS LABS LTD on March 4th, 2013.
Summary for RECLAST
| International Patents: | 26 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 128 |
| Clinical Trials: | 26 |
| Patent Applications: | 2,772 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for RECLAST |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for RECLAST |
| What excipients (inactive ingredients) are in RECLAST? | RECLAST excipients list |
| DailyMed Link: | RECLAST at DailyMed |

Recent Clinical Trials for RECLAST
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Australian and New Zealand Intensive Care Research Centre | Phase 2 |
| University of Nebraska | Phase 1/Phase 2 |
| San Francisco VA Health Care System | Phase 4 |
Pharmacology for RECLAST
| Drug Class | Bisphosphonate |
Anatomical Therapeutic Chemical (ATC) Classes for RECLAST
Paragraph IV (Patent) Challenges for RECLAST
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| RECLAST | Injection | zoledronic acid | 0.05 mg/mL, 100 mL vial | 021817 | 1 | 2008-08-29 |
US Patents and Regulatory Information for RECLAST
RECLAST is protected by one US patents.
Patents protecting RECLAST
Pharmaceutical products comprising bisphosphonates
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sandoz | RECLAST | zoledronic acid | INJECTABLE;INTRAVENOUS | 021817-001 | Apr 16, 2007 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for RECLAST
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sandoz | RECLAST | zoledronic acid | INJECTABLE;INTRAVENOUS | 021817-001 | Apr 16, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sandoz | RECLAST | zoledronic acid | INJECTABLE;INTRAVENOUS | 021817-001 | Apr 16, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for RECLAST
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Accord Healthcare S.L.U. | Zoledronic Acid Accord | zoledronic acid | EMEA/H/C/002667 Prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.Treatment of adult patients with tumour-induced hypercalcaemia (TIH). |
Authorised | yes | no | no | 2014-01-16 | |
| Actavis Group PTC ehf | Zoledronic acid Actavis | zoledronic acid | EMEA/H/C/002488 Prevention of skeletal-related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.Treatment of adult patients with tumour-induced hypercalcaemia. |
Authorised | yes | no | no | 2012-04-20 | |
| Mylan Pharmaceuticals Limited | Zoledronic acid Mylan | zoledronic acid | EMEA/H/C/002482 Prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone;treatment of adult patients with tumour-induced hypercalcaemia (TIH). |
Authorised | yes | no | no | 2012-08-23 | |
| Pfizer Europe MA EEIG | Zoledronic Acid Hospira | zoledronic acid | EMEA/H/C/002365 4 mg / 5 ml and 4 mg / 100 ml:Prevention of skeletal-related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.Treatment of adult patients with tumour-induced hypercalcaemia (TIH).5 mg / 100 ml:Treatment of osteoporosis:in post-menopausal women;in men;at increased risk of fracture, including those with a recent low-trauma hip fracture.Treatment of osteoporosis associated with long-term systemic glucocorticoid therapy:in post-menopausal women;in men;at increased risk of fracture.Treatment of Paget's disease of the bone in adults. |
Authorised | yes | no | no | 2012-11-19 | |
| Teva B.V. | Zoledronic acid Teva | zoledronic acid | EMEA/H/C/002439 Prevention of skeletal-related events and treatment of tumour-induced hypercalcaemia. |
Authorised | yes | no | no | 2012-08-16 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for RECLAST
See the table below for patents covering RECLAST around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | S63150291 | SUBSTITUTED ALKANEDIPHOSPHONIC ACID | ⤷ Try a Trial |
| Luxembourg | 90838 | ⤷ Try a Trial | |
| Canada | 1338937 | METHODE POUR LA PREPARATION DE NOUVEAUX DERIVES DE SUBSTITUTION D'ACIDES ALCANEDIPHOSPHONIQUES (PROCESS FOR THE MANUFACTURE OF NOVEL SUBSTITUTED ALKANEDIPHOSPHONIC ACIDS) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 0197788 | ⤷ Try a Trial | |
| China | 1272013 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for RECLAST
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0275821 | C300058 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: ZOLEDRONINEZUUR, DESGEWENST IN DE VORM VAN EEN ZOUT OF SOLVAAT, IN HET BIJZONDER ZOLEDRONINE-ZUUR-MONOHYDRAAT; REGISTRATION NO/DATE: EU/1/01/176/001-003 20010320 |
| 1591122 | 92174 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: ACIDE ZOLEDRONIQUE OU SON SEL PHARMACEUTIQUEMENT ACCEPTABLE OU TOUT HYDRATE DE CELUI-CI |
| 0275821 | 27/2001 | Austria | ⤷ Try a Trial | PRODUCT NAME: ZOLEDRONSAEURE UND DEREN SALZE UND HYDRATE; NAT. REGISTRATION NO/DATE: EU/1/01/176/001-003 20010320; FIRST REGISTRATION: LI 55463.01 20001128 |
| 1591122 | C300582 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: ZOLEDRONZUUR, DAN WEL EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, DAN WEL IEDER HYDRAAT DAARVAN; REGISTRATION NO/DATE: C(2007)4619 20071003 |
| 0275821 | SPC/GB01/039 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ZOLEDRONIC ACID OPTIONALLY IN THE FORM OF A SALT THEREOF; NATIONAL REGISTRATION NO/DATE: EU/1/01/176/001-003 20010320; FIRST REGISTRATION: CH 55463 20001128 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


