PROCHLORPERAZINE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Prochlorperazine, and what generic alternatives are available?
Prochlorperazine is a drug marketed by Alpharma Us Pharms, Baxter Hlthcare, Able, Cosette, Watson Labs, Morton Grove, Amneal, Avet Lifesciences, Caplin, Eugia Pharma, Gland Pharma Ltd, Hikma, Hospira, Marsam Pharms Llc, Mylan Labs Ltd, Nexus, Sagent, Smith And Nephew, Teva Parenteral, Viwit Pharm, Wyeth Ayerst, Bionpharma, Chartwell Rx, Duramed Pharms Barr, Glenmark Pharms Ltd, Ivax Sub Teva Pharms, Mylan, Novitium Pharma, Teva Pharms, and Zydus. and is included in forty-three NDAs.
The generic ingredient in PROCHLORPERAZINE is prochlorperazine maleate. There are twenty-one drug master file entries for this compound. Twenty-one suppliers are listed for this compound. Additional details are available on the prochlorperazine maleate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Prochlorperazine
A generic version of PROCHLORPERAZINE was approved as prochlorperazine maleate by NOVITIUM PHARMA on June 13th, 2022.
Summary for PROCHLORPERAZINE
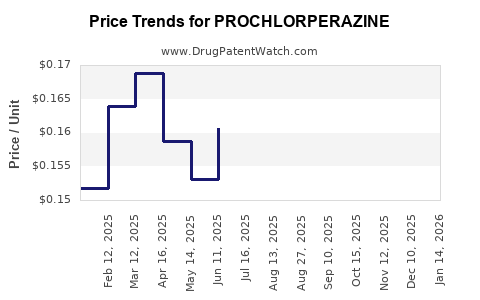
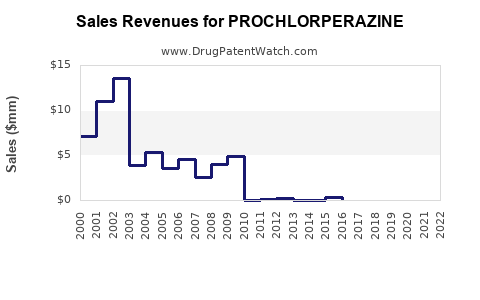
See drug prices for PROCHLORPERAZINE
Recent Clinical Trials for PROCHLORPERAZINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Calgary | Phase 3 |
| Ehsan Malek | Early Phase 1 |
| New York State Psychiatric Institute | Phase 2 |
Pharmacology for PROCHLORPERAZINE
| Drug Class | Phenothiazine |
Medical Subject Heading (MeSH) Categories for PROCHLORPERAZINE
Anatomical Therapeutic Chemical (ATC) Classes for PROCHLORPERAZINE
US Patents and Regulatory Information for PROCHLORPERAZINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duramed Pharms Barr | PROCHLORPERAZINE MALEATE | prochlorperazine maleate | TABLET;ORAL | 089484-001 | Jan 20, 1987 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Morton Grove | PROCHLORPERAZINE EDISYLATE | prochlorperazine edisylate | SYRUP;ORAL | 088597-001 | Oct 25, 1984 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mylan | PROCHLORPERAZINE MALEATE | prochlorperazine maleate | TABLET;ORAL | 040185-002 | Oct 28, 1996 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


