OSENI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Oseni, and what generic alternatives are available?
Oseni is a drug marketed by Takeda Pharms Usa and is included in one NDA. There are four patents protecting this drug.
This drug has one hundred and one patent family members in forty-two countries.
The generic ingredient in OSENI is alogliptin benzoate; pioglitazone hydrochloride. There are ten drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the alogliptin benzoate; pioglitazone hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Oseni
Oseni was eligible for patent challenges on January 25, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 4, 2029. This may change due to patent challenges or generic licensing.
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for OSENI
| International Patents: | 101 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 2 |
| Patent Applications: | 29 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for OSENI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for OSENI |
| What excipients (inactive ingredients) are in OSENI? | OSENI excipients list |
| DailyMed Link: | OSENI at DailyMed |
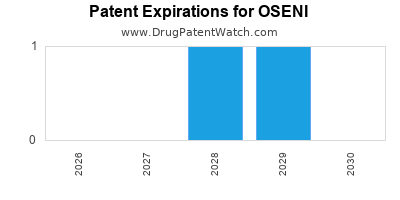

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for OSENI
Generic Entry Date for OSENI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for OSENI
Anatomical Therapeutic Chemical (ATC) Classes for OSENI
US Patents and Regulatory Information for OSENI
OSENI is protected by four US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of OSENI is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting OSENI
Dipeptidyl peptidase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING DIABETES COMPRISING ADMINISTERING ALOGLIPTIN
Dipeptidyl peptidase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING DIABETES COMPRISING ADMINISTERING A COMPOUND SUCH AS ALOGLIPTIN
Dipeptidyl peptidase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Solid preparation comprising alogliptin and pioglitazone
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Expired US Patents for OSENI
International Patents for OSENI
When does loss-of-exclusivity occur for OSENI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5097
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 08211981
Estimated Expiration: ⤷ Try a Trial
Austria
Patent: 88227
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0807453
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 77201
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 08000279
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1646420
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 992
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0110094
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 11264
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 07905
Estimated Expiration: ⤷ Try a Trial
Dominican Republic
Patent: 009000195
Estimated Expiration: ⤷ Try a Trial
Ecuador
Patent: 099608
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 5180
Estimated Expiration: ⤷ Try a Trial
Patent: 0970726
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 07905
Estimated Expiration: ⤷ Try a Trial
Georgia, Republic of
Patent: 0125410
Estimated Expiration: ⤷ Try a Trial
Germany
Patent: 2008003522
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 38188
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 0108
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 84968
Estimated Expiration: ⤷ Try a Trial
Patent: 10517937
Estimated Expiration: ⤷ Try a Trial
Jordan
Patent: 50
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 7596
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 09008100
Estimated Expiration: ⤷ Try a Trial
Montenegro
Patent: 239
Estimated Expiration: ⤷ Try a Trial
Morocco
Patent: 169
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 9008
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 081663
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 07905
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 07905
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 592
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 07905
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0905621
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1486091
Estimated Expiration: ⤷ Try a Trial
Patent: 090109115
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 54397
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 53041
Estimated Expiration: ⤷ Try a Trial
Patent: 0836775
Estimated Expiration: ⤷ Try a Trial
Patent: 1350143
Estimated Expiration: ⤷ Try a Trial
Tunisia
Patent: 09000317
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 828
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering OSENI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Denmark | 1084705 | ⤷ Try a Trial | |
| European Patent Office | 0749751 | ⤷ Try a Trial | |
| Norway | 313226 | ⤷ Try a Trial | |
| Japan | 2002501889 | ⤷ Try a Trial | |
| Malaysia | 146290 | DIPEPTIDYL PEPTIDASE INHIBITORS | ⤷ Try a Trial |
| Hong Kong | 1019204 | ⤷ Try a Trial | |
| Germany | 122007000038 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for OSENI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1084705 | C300708 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LINAGLIPTIN; REGISTRATION NO/DATE: EU/1/11/707/001-011 20111224 |
| 1586571 | CR 2014 00011 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ALOGLIPTIN; REG. NO/DATE: EU/1/13/844/001-027 20130923 |
| 0896538 | 91382 | Luxembourg | ⤷ Try a Trial | 91382, EXPIRES: 20220424 |
| 1084705 | PA2014042 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: VILDAGLIPTINUM; REGISTRATION NO/DATE: EU/1/07/414/001-010, 2007 09 26 EU/1/07/414/018 20070926 |
| 1586571 | CA 2014 00011 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ALOGLIPTIN; REG. NO/DATE: EU/1/13/844/001-027 20130919 |
| 1084705 | SPC/GB14/083 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ALOGLIPTIN; REGISTERED: UK EU/1/13/844 20130923 |
| 1084705 | C01084705/03 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTIN; REGISTRATION NO/DATE: SWISSMEDIC 59390 05.02.2010 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


