NEVANAC Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Nevanac, and when can generic versions of Nevanac launch?
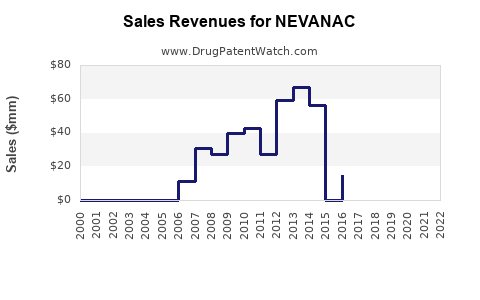
Nevanac is a drug marketed by Harrow Eye and is included in one NDA. There are three patents protecting this drug.
This drug has twenty-seven patent family members in twenty-three countries.
The generic ingredient in NEVANAC is nepafenac. There are eight drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the nepafenac profile page.
DrugPatentWatch® Generic Entry Outlook for Nevanac
Nevanac was eligible for patent challenges on August 19, 2009.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 2, 2025. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for NEVANAC
| International Patents: | 27 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 119 |
| Clinical Trials: | 25 |
| Patent Applications: | 2,245 |
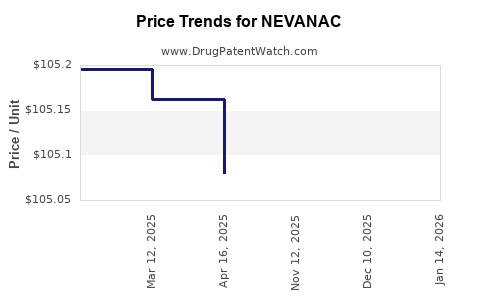
| Drug Prices: | Drug price information for NEVANAC |
| What excipients (inactive ingredients) are in NEVANAC? | NEVANAC excipients list |
| DailyMed Link: | NEVANAC at DailyMed |
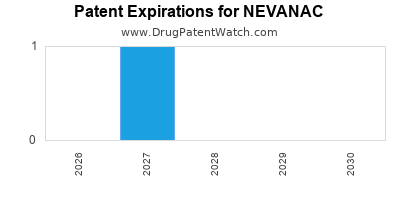
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for NEVANAC
Generic Entry Date for NEVANAC*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SUSPENSION/DROPS;OPHTHALMIC |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for NEVANAC
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Kafrelsheikh University | Phase 4 |
| Ifocus Oyeklinikk | Phase 4 |
| The Research Council of Norway | Phase 4 |
Pharmacology for NEVANAC
| Drug Class | Nonsteroidal Anti-inflammatory Drug |
| Mechanism of Action | Cyclooxygenase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for NEVANAC
US Patents and Regulatory Information for NEVANAC
NEVANAC is protected by three US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of NEVANAC is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting NEVANAC
Topical nepafenac formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING OCULAR INFLAMMATION
Topical nepafenac formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Topical nepafenac formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Harrow Eye | NEVANAC | nepafenac | SUSPENSION/DROPS;OPHTHALMIC | 021862-001 | Aug 19, 2005 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Harrow Eye | NEVANAC | nepafenac | SUSPENSION/DROPS;OPHTHALMIC | 021862-001 | Aug 19, 2005 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Harrow Eye | NEVANAC | nepafenac | SUSPENSION/DROPS;OPHTHALMIC | 021862-001 | Aug 19, 2005 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for NEVANAC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Harrow Eye | NEVANAC | nepafenac | SUSPENSION/DROPS;OPHTHALMIC | 021862-001 | Aug 19, 2005 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for NEVANAC
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Novartis Europharm Limited | Nevanac | nepafenac | EMEA/H/C/000818 Nevanac is indicated for:, , , prevention and treatment of postoperative pain and inflammation associated with cataract surgery;, reduction in the risk of postoperative macular oedema associated with cataract surgery in diabetic patients., , |
Authorised | no | no | no | 2007-12-11 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for NEVANAC
When does loss-of-exclusivity occur for NEVANAC?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2252
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 05311738
Estimated Expiration: ⤷ Try a Trial
Austria
Patent: 76200
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0518904
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 86807
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1068573
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 10780
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 19362
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 19362
Estimated Expiration: ⤷ Try a Trial
Germany
Patent: 2005022756
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 04225
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 68844
Estimated Expiration: ⤷ Try a Trial
Patent: 08521926
Estimated Expiration: ⤷ Try a Trial
Patent: 12041368
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 07006558
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 19362
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 19362
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 07124638
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 19362
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0704763
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1289661
Estimated Expiration: ⤷ Try a Trial
Patent: 070089687
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 48249
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 58290
Estimated Expiration: ⤷ Try a Trial
Patent: 0626132
Estimated Expiration: ⤷ Try a Trial
Uruguay
Patent: 238
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering NEVANAC around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Germany | 602005022756 | ⤷ Try a Trial | |
| Spain | 2171543 | ⤷ Try a Trial | |
| China | 1129397 | ⤷ Try a Trial | |
| Mexico | 2007006558 | FORMULACIONES TOPICAS DE NEPAFENAC. (TOPICAL NEPAFENAC FORMULATIONS.) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2006060618 | ⤷ Try a Trial | |
| Hong Kong | 1012554 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for NEVANAC
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0999825 | 92301 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: NEPAFENAC-SUSPENSION OPHTALMIQUE |
| 0716600 | C00716600/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: NEPAFENAC; REGISTRATION NUMBER/DATE: SWISSMEDIC 58745 24.09.2008 |
| 0999825 | C300622 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: NEPAFENAC; REGISTRATION NO/DATE: EU/1/07/433/002 20130503 |
| 0999825 | 122013000085 | Germany | ⤷ Try a Trial | PRODUCT NAME: NEPAFENAC (OPHTHALMISCHE SUSPENSION); REGISTRATION NO/DATE: EU 1/07/433/002 20130503 |
| 0999825 | CA 2013 00055 | Denmark | ⤷ Try a Trial | PRODUCT NAME: NEPAFENAC (3 MG/ML), HERUNDER NEPAFENAC I KOMBINATION MED GLACTOMANNANPOLYMERER, ISAER 3 MG/ML NEPAFENAC I KOMBINATION MED GALACTOMANNANPOLYMERER, SAMT OFTALMISKE SAMMENSAETNINGER DERAF; REG. NO/DATE: EU1/07/433/002 20130503 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


