MOVIPREP Drug Patent Profile
✉ Email this page to a colleague
When do Moviprep patents expire, and what generic alternatives are available?
Moviprep is a drug marketed by Salix Pharms and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has fifty-four patent family members in twenty-two countries.
The generic ingredient in MOVIPREP is ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate. There are six drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate profile page.
DrugPatentWatch® Generic Entry Outlook for Moviprep
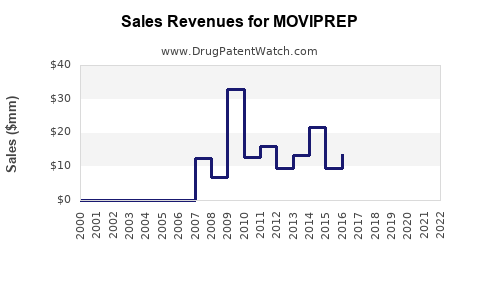
Annual sales in 2021 were $646k (peak sales were $33mm in 2009).
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for MOVIPREP
| International Patents: | 54 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Clinical Trials: | 40 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for MOVIPREP |
| Drug Sales Revenues: | Drug sales revenues for MOVIPREP |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for MOVIPREP |
| What excipients (inactive ingredients) are in MOVIPREP? | MOVIPREP excipients list |
| DailyMed Link: | MOVIPREP at DailyMed |
Recent Clinical Trials for MOVIPREP
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Aalborg University Hospital | Phase 4 |
| North Denmark Regional Hospital | Phase 4 |
| Radboud University Medical Center | N/A |
Anatomical Therapeutic Chemical (ATC) Classes for MOVIPREP
Paragraph IV (Patent) Challenges for MOVIPREP
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| MOVIPREP | For Oral Solution | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | 100 g, 7.5 g, 2.691 g, 1.015 g, 5.9 g and 4.7 g per pouch | 021881 | 1 | 2007-11-27 |
US Patents and Regulatory Information for MOVIPREP
MOVIPREP is protected by two US patents.
Patents protecting MOVIPREP
Colon cleansing compositions and methods
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Colon cleansing compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salix Pharms | MOVIPREP | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | FOR SOLUTION;ORAL | 021881-001 | Aug 2, 2006 | AA | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Salix Pharms | MOVIPREP | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | FOR SOLUTION;ORAL | 021881-001 | Aug 2, 2006 | AA | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for MOVIPREP
See the table below for patents covering MOVIPREP around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2009105770 | СУХАЯ КОМПОЗИЦИЯ ДЛЯ СМЕШИВАНИЯ С ВОДОЙ (ВАРИАНТЫ), ОЧИСТИТЕЛЬНЫЙ ПРЕПАРАТ И НАБОР КОМПОНЕНТОВ ДЛЯ ОЧИСТКИ ТОЛСТОЙ КИШКИ И СПОСОБ ОЧИЩЕНИЯ ТОЛСТОЙ КИШКИ (ВАРИАНТЫ) | ⤷ Try a Trial |
| United Kingdom | 0224909 | ⤷ Try a Trial | |
| Denmark | 2014304 | ⤷ Try a Trial | |
| Russian Federation | 2005116023 | СУХАЯ КОМПОЗИЦИЯ ДЛЯ СМЕШИВАНИЯ С ВОДОЙ (ВАРИАНТЫ), ОЧИСТИТЕЛЬНЫЙ ПРЕПАРАТ И НАБОР КОМПОНЕНТОВ ДЛЯ ОЧИСТКИ ТОЛСТОЙ КИШКИ, ПРИМЕНЕНИЕ ПОЛИЭТИЛЕНГЛИКОЛЯ В КАЧЕСТВЕ КОМПОНЕНТА ЭТИХ СРЕДСТВ (ВАРИАНТЫ) И СПОСОБ ОЧИЩЕНИЯ ТОЛСТОЙ КИШКИ (ВАРИАНТЫ) | ⤷ Try a Trial |
| Slovenia | 1567193 | ⤷ Try a Trial | |
| European Patent Office | 2314319 | Compositions pour le nettoyage du colon (Colon Cleansing Compositions) | ⤷ Try a Trial |
| Slovenia | 2014304 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for MOVIPREP
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3141251 | 132021000000044 | Italy | ⤷ Try a Trial | PRODUCT NAME: UN PRODOTTO MEDICINALE COSTITUITO DA UNA COMBINAZIONE DI UNA PRIMA DOSE DI COMPOSIZIONE FARMACEUTICA E UNA SECONDA DOSE DI COMPOSIZIONE FARMACEUTICA, LA PRIMA DOSE DI COMPOSIZIONE FARMACEUTICA E COSTITUITA DAGLI INGREDIENTI ATTIVI POLIETILENGLICOLE, SOLFATO DI SODIO, CLORURO DI SODIO E CLORURO DI POTASSIO E LA SECONDA DOSE DI COMPOSIZIONE FARMACEUTICA E COSTITUITA DAGLI INGREDIENTI ATTIVI POLIETILENGLICOLE, ACIDO ASCORBICO, ASCORBATO DI SODIO, CLORURO DI SODIO E CLORURO DI POTASSIO.(PLENVU); AUTHORISATION NUMBER(S) AND DATE(S): IS/1/17/083/01, 20171016;045671017, 045671029, 045671031, 045671043, 045671056, 20171213 |
| 3141251 | C202130017 | Spain | ⤷ Try a Trial | PRODUCT NAME: COMBINACION DE PRINCIPIOS ACTIVOS EN DOS DOSIS, EN LA QUE LA PRIMERA DOSIS CONSISTE EN LOS PRINCIPIOS ACTIVOS POLIETILENGLICOL, SULFATO DE SODIO, CLORURO DE SODIO Y CLORURO DE POTASIO, Y LA SEGUNDA DOSIS CONSISTE EN LOS PRINCIPIOS ACTIVOS POLIETILENGLICOL, ACIDO ASCORBICO, ASCORBATO DE SODIO, CLORURO DE SODIO Y CLORURO DE POTASIO.; NATIONAL AUTHORISATION NUMBER: 82959-SE/H/1801/001/DC; DATE OF AUTHORISATION: 20180525; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): IS/1/17/083/01; DATE OF FIRST AUTHORISATION IN EEA: 20171016 |
| 3141251 | 301099 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: A MEDICINAL PRODUCT CONSISTING OF A COMBINATION OF A FIRST DOSE PHARMACEUTICAL COMPOSITION AND A SECOND DOSE PHARMACEUTICAL COMPOSITION, THE FIRST DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, SODIUM SULPHATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE AND THE SECOND DOSE PHARMACEUTICAL COMPOSITION CONSISTING OF THE ACTIVE INGREDIENTS POLYETHYLENE GLYCOL, ASCORBIC ACID, SODIUM ASCORBATE, SODIUM CHLORIDE AND POTASSIUM CHLORIDE; NATIONAL REGISTRATION NO/DATE: RVG 120195 20171114; FIRST REGISTRATION: IS IS/1/17/083/01 20171016 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


