MINIVELLE Drug Patent Profile
✉ Email this page to a colleague
When do Minivelle patents expire, and when can generic versions of Minivelle launch?
Minivelle is a drug marketed by Noven and is included in one NDA. There are four patents protecting this drug and two Paragraph IV challenges.
This drug has twenty-three patent family members in fifteen countries.
The generic ingredient in MINIVELLE is estradiol. There are seventy-five drug master file entries for this compound. Forty-five suppliers are listed for this compound. Additional details are available on the estradiol profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Minivelle
A generic version of MINIVELLE was approved as estradiol by BARR LABS INC on October 22nd, 1997.
Summary for MINIVELLE
| International Patents: | 23 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 102 |
| Formulation / Manufacturing: | see details |
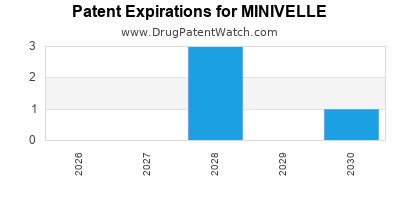
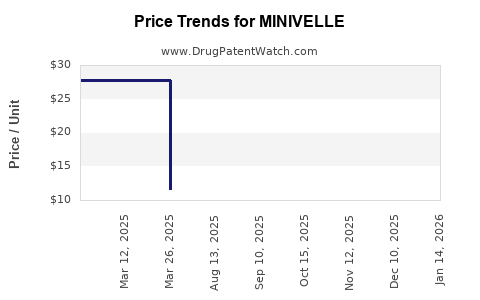
| Drug Prices: | Drug price information for MINIVELLE |
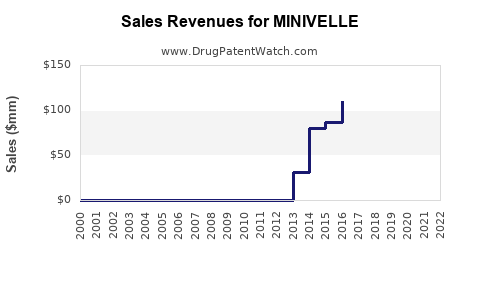
| Drug Sales Revenues: | Drug sales revenues for MINIVELLE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for MINIVELLE |
| What excipients (inactive ingredients) are in MINIVELLE? | MINIVELLE excipients list |
| DailyMed Link: | MINIVELLE at DailyMed |
Pharmacology for MINIVELLE
| Drug Class | Estrogen |
| Mechanism of Action | Estrogen Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for MINIVELLE
US Patents and Regulatory Information for MINIVELLE
MINIVELLE is protected by four US patents.
Patents protecting MINIVELLE
Transdermal estrogen device and delivery
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Transdermal estrogen device and delivery
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Transdermal estrogen device and delivery
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR ADMINISTERING ESTRADIOL COMPRISING A MONOLITHIC TRANSDERMAL DRUG DELIVERY SYSTEM CONSISTING OF (I) A BACKING LAYER AND (II) A SINGLE ADHESIVE POLYMER MATRIX LAYER AS CLAIMED IN US PATENT NO. 9730900
Transdermal estrogen device and delivery
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-005 | Sep 23, 2014 | AB3 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-001 | Oct 29, 2012 | AB3 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-005 | Sep 23, 2014 | AB3 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-002 | Oct 29, 2012 | AB3 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-003 | Oct 29, 2012 | AB3 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-004 | Oct 29, 2012 | AB3 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-004 | Oct 29, 2012 | AB3 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for MINIVELLE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-001 | Oct 29, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-005 | Sep 23, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-002 | Oct 29, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-001 | Oct 29, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-001 | Oct 29, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-002 | Oct 29, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-002 | Oct 29, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for MINIVELLE
See the table below for patents covering MINIVELLE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Singapore | 77626 | Compositions and methods for topical administration of pharmaceutically active agents | ⤷ Try a Trial |
| European Patent Office | 2310001 | DISPOSITIF D'ADMINISTRATION TRANSDERMIQUE D'ESTRADIOL (TRANSDERMAL ESTRADIOL DEVICE AND DELIVERY) | ⤷ Try a Trial |
| South Korea | 100391229 | ⤷ Try a Trial | |
| Russian Federation | 2526186 | СРЕДСТВО И СПОСОБ ТРАНСДЕРМАЛЬНОЙ ДОСТАВКИ ЭСТРОГЕНА (AGENT AND METHOD FOR TRANSDERMAL DELIVERY OF OESTROGEN) | ⤷ Try a Trial |
| Austria | 99176 | ⤷ Try a Trial | |
| Israel | 112269 | Adhesive compositions for use in transdermal drug delivery systems and method of their preparation | ⤷ Try a Trial |
| New Zealand | 278769 | TRANSDERMAL DEVICE USING POLYVINYLPYRROLIDONE AS A SOLUBILITY ENHANCER | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for MINIVELLE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0334429 | 97C0002 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL; NAT. REGISTRATION NO/DATE: NL 18978 19960731; FIRST REGISTRATION: SE - 11783 19930305 |
| 2782584 | C202130068 | Spain | ⤷ Try a Trial | PRODUCT NAME: COMPOSICION QUE CONTIENE ESTRADIOL (17BETA-ESTRADIOL), INCLUYENDO EN FORMA DE HEMIHIDRATO, Y PROGESTERONA; NATIONAL AUTHORISATION NUMBER: 85988-NL/H/4994/001/DC; DATE OF AUTHORISATION: 20210528; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): BE582231; DATE OF FIRST AUTHORISATION IN EEA: 20210406 |
| 1453521 | 300814 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL EN ETHINYLESTRADIOL; NATIONAL REGISTRATION NO/DATE: RVG 117453 20151211; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 2782584 | 301153 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTAINING BOTH ESTRADIOL (17SS-ESTRADIOL), OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE OR SOLVATE THEREOF (INCLUDING IN HEMIHYDRATE FORM), AND PROGESTERONE; NATIONAL REGISTRATION NO/DATE: RVG 125821 20210611; FIRST REGISTRATION: BE BE582231 20210406 |
| 0136011 | C00136011/03 | Switzerland | ⤷ Try a Trial | PRODUCT NMAE: ESTRADIOL UND MEDROXYPROGESTERONACETAT; REGISTRATION NO/DATE: IKS 55288 20000417 |
| 0771217 | 07C0001 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL BETADEX CLATHRATE; NAT. REGISTRATION NO/DATE: NL 32343 20060710; FIRST REGISTRATION: NL - RVG 31781 20050804 |
| 0770388 | SPC/GB09/026 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL AND COMBINATIONS OF ESTRADIOL AND DIENOGEST, PREFERABLY ESTRADIOL VALERATE AND COMBINATIONS OF ESTRADIOL VALERATE AND DIENOGEST; REGISTERED: BE BE 327792 20081103; UK PL 00010/0576-0001 20081208 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


