MICARDIS HCT Drug Patent Profile
✉ Email this page to a colleague
When do Micardis Hct patents expire, and when can generic versions of Micardis Hct launch?
Micardis Hct is a drug marketed by Boehringer Ingelheim and is included in one NDA.
The generic ingredient in MICARDIS HCT is hydrochlorothiazide; telmisartan. There are thirty-two drug master file entries for this compound. Nine suppliers are listed for this compound. Additional details are available on the hydrochlorothiazide; telmisartan profile page.
Summary for MICARDIS HCT
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 9 |
| Clinical Trials: | 71 |
| Patent Applications: | 48 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for MICARDIS HCT |
| What excipients (inactive ingredients) are in MICARDIS HCT? | MICARDIS HCT excipients list |
| DailyMed Link: | MICARDIS HCT at DailyMed |
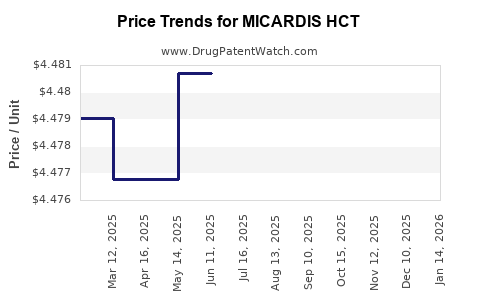
See drug prices for MICARDIS HCT
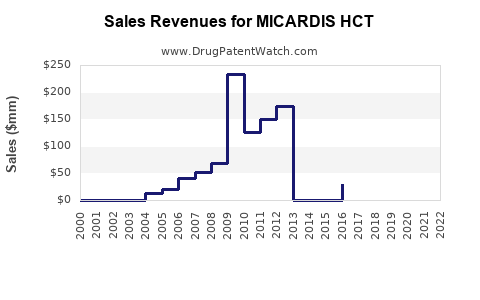
Recent Clinical Trials for MICARDIS HCT
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Hawaii | Phase 2 |
| Queens Medical Center | Phase 2 |
| HK inno.N Corporation | Phase 1 |
Pharmacology for MICARDIS HCT
| Drug Class | Angiotensin 2 Receptor Blocker Thiazide Diuretic |
| Mechanism of Action | Angiotensin 2 Receptor Antagonists |
| Physiological Effect | Increased Diuresis |
Anatomical Therapeutic Chemical (ATC) Classes for MICARDIS HCT
Paragraph IV (Patent) Challenges for MICARDIS HCT
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| MICARDIS HCT | Tablets | hydrochlorothiazide; telmisartan | 80 mg/12.5 mg and 40 mg/12.5 mg | 021162 | 1 | 2008-12-31 |
US Patents and Regulatory Information for MICARDIS HCT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | MICARDIS HCT | hydrochlorothiazide; telmisartan | TABLET;ORAL | 021162-001 | Nov 17, 2000 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Boehringer Ingelheim | MICARDIS HCT | hydrochlorothiazide; telmisartan | TABLET;ORAL | 021162-002 | Nov 17, 2000 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Boehringer Ingelheim | MICARDIS HCT | hydrochlorothiazide; telmisartan | TABLET;ORAL | 021162-003 | Apr 19, 2004 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for MICARDIS HCT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | MICARDIS HCT | hydrochlorothiazide; telmisartan | TABLET;ORAL | 021162-002 | Nov 17, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| Boehringer Ingelheim | MICARDIS HCT | hydrochlorothiazide; telmisartan | TABLET;ORAL | 021162-002 | Nov 17, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| Boehringer Ingelheim | MICARDIS HCT | hydrochlorothiazide; telmisartan | TABLET;ORAL | 021162-001 | Nov 17, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| Boehringer Ingelheim | MICARDIS HCT | hydrochlorothiazide; telmisartan | TABLET;ORAL | 021162-003 | Apr 19, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| Boehringer Ingelheim | MICARDIS HCT | hydrochlorothiazide; telmisartan | TABLET;ORAL | 021162-001 | Nov 17, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for MICARDIS HCT
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Krka, d.d., Novo mesto | Tolucombi | telmisartan, hydrochlorothiazide | EMEA/H/C/002549 Tolucombi fixed-dose combination (80 mg telmisartan/25 mg hydrochlorothiazide) is indicated in adults whose blood pressure is not adequately controlled on Tolucombi 80 mg/12.5 mg (80 mg telmisartan/12.5 mg hydrochlorothiazide) or adults who have been previously stabilised on telmisartan and hydrochlorothiazide given separately. |
Authorised | yes | no | no | 2013-03-13 | |
| Bayer AG | Kinzalkomb | telmisartan, hydrochlorothiazide | EMEA/H/C/000415 Treatment of essential hypertension.Kinzalkomb fixed-dose combination (40 mg telmisartan / 12.5 mg hydrochlorothiazide, 80 mg telmisartan / 12.5 mg hydrochlorothiazide) is indicated in patients whose blood pressure is not adequately controlled on telmisartan alone.Kinzalkomb fixed-dose combination (80 mg telmisartan / 25 mg hydrochlorothiazide) is indicated in patients whose blood pressure is not adequately controlled on Kinzalkomb (80 mg telmisartan / 12.5 mg hydrochlorothiazide) or patients who have been previously stabilised on telmisartan and hydrochlorothiazide given separately. |
Authorised | no | no | no | 2002-04-19 | |
| Bayer AG | PritorPlus | telmisartan, hydrochlorothiazide | EMEA/H/C/000414 Treatment of essential hypertension.PritorPlus fixed-dose combination (40 mg telmisartan / 12.5 mg hydrochlorothiazide, 80mg telmisartan / 12.5 mg hydrochlorothiazide) is indicated in patients whose blood pressure is not adequately controlled on telmisartan alone.PritorPlus fixed-dose combination (80 mg telmisartan / 25 mg hydrochlorothiazide) is indicated in patients whose blood pressure is not adequately controlled on PritorPlus (80 mg telmisartan / 12.5 mg hydrochlorothiazide) or patients who have been previously stabilised on telmisartan and hydrochlorothiazide given separately. |
Authorised | no | no | no | 2002-04-22 | |
| Actavis Group hf | Actelsar HCT | telmisartan, hydrochlorothiazide | EMEA/H/C/002676 Treatment of essential hypertension.Actelsar HCT fixed-dose combination (40 mg telmisartan / 12.5 mg hydrochlorothiazide) is indicated in adults whose blood pressure is not adequately controlled on telmisartan alone.Actelsar HCT fixed-dose combination (80 mg telmisartan / 12.5 mg hydrochlorothiazide) is indicated in adults whose blood pressure is not adequately controlled on telmisartan alone.Actelsar HCT fixed-dose combination (80 mg telmisartan / 25 mg hydrochlorothiazide) is indicated in adults whose blood pressure is not adequately controlled on Actelsar HCT 80 mg / 12.5 mg (80 mg telmisartan / 12.5 mg hydrochlorothiazide) or adults who have been previously stabilised on telmisartan and hydrochlorothiazide given separately. |
Authorised | yes | no | no | 2013-03-13 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for MICARDIS HCT
See the table below for patents covering MICARDIS HCT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Slovakia | 32493 | BENZIMIDAZOLE DERIVATIVES, PROCESS FOR THEIR PREPARATION AND PHARMACEUTICAL COMPOSITIONS WITH THEIR CONTENTS | ⤷ Try a Trial |
| New Zealand | 245741 | 1-BIPHENYLYLMETHYLBENZIMIDAZOLE DERIVATIVES; PHARMACEUTICAL COMPOSITIONS AND PREPARATORY METHODS | ⤷ Try a Trial |
| Taiwan | 229207 | ⤷ Try a Trial | |
| Taiwan | 239138 | ⤷ Try a Trial | |
| Singapore | 50481 | Benzimidazoles pharmaceutical compositions containing these compounds and processes for preparing them | ⤷ Try a Trial |
| Slovakia | 285429 | ⤷ Try a Trial | |
| Germany | 4117121 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for MICARDIS HCT
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0480717 | 98C0025 | Belgium | ⤷ Try a Trial | PRODUCT NAME: LOSARTAN POTASSIUM; HYDROCHLOROTHIAZIDE; NAT. REGISTRATION NO/DATE: NL 20 037 19950215; FIRST REGISTRATION: FR - NL 20 037 19950215 |
| 0502314 | 1190006-5.L | Sweden | ⤷ Try a Trial | PRODUCT NAME: TELMISARTAN, VALFRITT I FORM AV FARMACEUTISKT GODTAGBARA SALT, OCH AMLODIPIN, VALFRITT I FORM AV FARMACEUTISKT GODTAGBARA SALT SAERSKILT AMOLODIPINBESILAT; REG. NO/DATE: EU/1/10/648/001-028 " EESGODKLANDKOD="EG" EESGODKDATUM="2010-10-07" SEGODKNR="EU/1/10/648/001-028 " SEGODKLANDKOD="EG" SEGODKDATUM="2010-10-07" FLAG="L" SPCNR="1190006-5" 20101007 |
| 0502314 | 99C0014 | Belgium | ⤷ Try a Trial | PRODUCT NAME: TELMISARTAN REGISTRATION NO/DATE: EU/1/98/089/001 / 19981211 |
| 0502314 | C990007 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: TELMISARTAN, DESGEWENST IN DE VORM VAN EEN FYSIOLOGISCH VERDRAAGBAAR ZOUT; REGISTRATION NO/DATE: EU/1/98/089/001-EU/1/98/089/010 19981211 |
| 0502314 | SPC/GB99/014 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TELMISARTAN, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT; REGISTERED: UK EU/1/98/089/001 19981211; UK EU/1/98/089/002 19981211; UK EU/1/98/089/003 19981211; UK EU/1/98/089/004 19981211; UK EU/1/98/089/005 19981211; UK EU/1/98/089/006 19981211; UK EU/1/98/089/007 19981211; UK EU/1/98/089/008 19981211; UK EU/1/98/089/009 19981211; UK EU/1/98/089/010 19981211 |
| 0502314 | C300095 | Netherlands | ⤷ Try a Trial | PRODCUT NAME: TELMISARTAN, DESGEWENST IN DE VORM VAN EEN FYSIOLOGISCH VERDRAAGBAAR ZOUT, EN HYDROCHLOROTHIAZIDE; REGISTRATION NO/DATE: EU/1/02/213/001-010 20020419 |
| 0502314 | SPC/GB11/010 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: THE COMBINATION OF A) TELMISARTAN, OPTIONALLY IN THE FORM OF PHARMACEUTICALLY ACCEPTABLE SALTS, AND B) AMLODIPINE, OPTIONALLY IN THE FORM OF PHARMACEUTICALLY ACCEPTABLE SALTS, ESPECIALLY AMLODIPINE BESYLATE; REGISTERED: UK EU/1/10/648/001 20101007; UK EU/1/10/648/002 20101007; UK EU/1/10/648/003 20101007; UK EU/1/10/648/004 20101007; UK EU/1/10/648/005 20101007; UK EU/1/10/648/006 20101007; UK EU/1/10/648/007 20101007; UK EU/1/10/648/008 20101007; UK EU/1/10/648/009 20101007; UK EU/1/10/648/010 20101007; UK EU/1/10/648/011 20101007; UK EU/1/10/648/012 20101007; UK EU/1/10/648/013 20101007; UK EU/1/10/648/014 20101007; UK EU/1/10/648/015 20101007; UK EU/1/10/648/016 20101007; UK |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


