MEKINIST Drug Patent Profile
✉ Email this page to a colleague
When do Mekinist patents expire, and what generic alternatives are available?
Mekinist is a drug marketed by Novartis and is included in two NDAs. There are twelve patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and sixty-five patent family members in forty-five countries.
The generic ingredient in MEKINIST is trametinib dimethyl sulfoxide. There is one drug master file entry for this compound. One supplier is listed for this compound. Additional details are available on the trametinib dimethyl sulfoxide profile page.
DrugPatentWatch® Generic Entry Outlook for Mekinist
Mekinist was eligible for patent challenges on May 29, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 15, 2030. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for MEKINIST
| International Patents: | 165 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 104 |
| Clinical Trials: | 92 |
| Patent Applications: | 3,196 |
| Drug Prices: | Drug price information for MEKINIST |
| What excipients (inactive ingredients) are in MEKINIST? | MEKINIST excipients list |
| DailyMed Link: | MEKINIST at DailyMed |
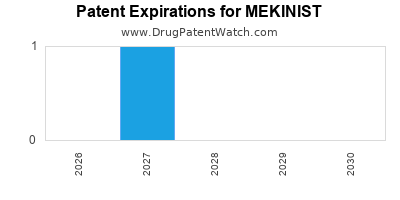

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for MEKINIST
Generic Entry Dates for MEKINIST*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
Generic Entry Dates for MEKINIST*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for MEKINIST
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Erasca, Inc. | Phase 1 |
| Peter Hosein, MD | Phase 1 |
| University of Miami Sylvester Comprehensive Cancer Center | Phase 1 |
Pharmacology for MEKINIST
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Protein Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for MEKINIST
Paragraph IV (Patent) Challenges for MEKINIST
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| MEKINIST | Tablets | trametinib dimethyl sulfoxide | 0.5 mg and 2 mg | 204114 | 1 | 2023-09-28 |
US Patents and Regulatory Information for MEKINIST
MEKINIST is protected by twelve US patents and eleven FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of MEKINIST is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting MEKINIST
Method of adjuvant cancer treatment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pyrimidine compound and medical use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pyrimidine compound and medical use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical composition
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical combination of MEK inhibitor and B-RAF inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: MEKINIST IS INDICATED, IN COMBINATION WITH DABRAFENIB, FOR THE TREATMENT OF PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER WITH LOW-GRADE GLIOMA (LGG) WITH A BRAF V600E MUTATION WHO REQUIRE SYSTEMIC THERAPY
Pharmaceutical combination of MEK inhibitor and B-RAF inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pyrimidine compound and medical use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: MEKINIST IS INDICATED, IN COMBINATION WITH DABRAFENIB, FOR THE TREATMENT OF PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER WITH LOW-GRADE GLIOMA (LGG) WITH A BRAF V600E MUTATION WHO REQUIRE SYSTEMIC THERAPY
Pyrimidine compound and medical use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical combination of MEK inhibitor and B-Raf inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical composition
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical composition
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical composition
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting MEKINIST
TREATMENT OF PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER WITH LOW-GRADE GLIOMA (LGG) WITH A BRAF V600E MUTATION WHO REQUIRE SYSTEMIC THERAPY
Exclusivity Expiration: ⤷ Try a Trial
NEW PRODUCT
Exclusivity Expiration: ⤷ Try a Trial
TRAMETINIB IS INDICATED, IN COMBINATION WITH DABRAFENIB, FOR THE ADJUVANT TREATMENT OF PATIENTS WITH MELANOMA WITH BRAF V600E OR V600K MUTATIONS, AS DETECTED BY AN FDA-APPROVED TEST, AND INVOLVEMENT OF LYMPH NODE(S), FOLLOWING COMPLETE RESECTION
Exclusivity Expiration: ⤷ Try a Trial
TRAMETINIB AND DABRAFENIB IN COMBINATION, FOR THE TREATMENT OF PATIENTS WITH LOCALLY ADVANCED OR METASTATIC ANAPLASTIC THYROID CANCER (ATC) WITH BRAF V600E MUTATION AND WITH NO SATISFACTORY LOCOREGIONAL TREATMENT OPTIONS
Exclusivity Expiration: ⤷ Try a Trial
TRAMETINIB IN COMBINATION WITH DABRAFENIB, FOR THE TX. OF PTS WITH METASTATIC NON-SMALL CELL LUNG CANCERWITH BRAF V600E MUTATION AS DETECTED BY AN FDA-APPROVED TEST
Exclusivity Expiration: ⤷ Try a Trial
TREATMENT OF PEDIATRIC PATIENTS 1 YEAR OF AGE AND OLDER WITH LOW-GRADE GLIOMA WITH A BRAF V600 MUTATION WHO REQUIRE SYSTEMIC THERAPY
Exclusivity Expiration: ⤷ Try a Trial
TRAMETINIB IS INDICATED IN COMBINATION WITH DABRAFENIB, FOR THE TREATMENT OF ADULT AND PEDIATRIC PATIENTS 6 YEARS OF AGE AND OLDER WITH UNRESECTABLE OR METASTATIC SOLID TUMORS WITH BRAF V600E MUTATION WHO HAVE PROGRESSED FOLLOWING PRIOR TREATMENT AND HAVE NO SATISFACTORY ALTERNATIVE TREATMENT OPTIONS
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | MEKINIST | trametinib dimethyl sulfoxide | TABLET;ORAL | 204114-002 | May 29, 2013 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novartis | MEKINIST | trametinib dimethyl sulfoxide | TABLET;ORAL | 204114-002 | May 29, 2013 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novartis | MEKINIST | trametinib dimethyl sulfoxide | TABLET;ORAL | 204114-003 | May 29, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novartis | MEKINIST | trametinib dimethyl sulfoxide | TABLET;ORAL | 204114-003 | May 29, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Novartis | MEKINIST | trametinib dimethyl sulfoxide | TABLET;ORAL | 204114-003 | May 29, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novartis | MEKINIST | trametinib dimethyl sulfoxide | TABLET;ORAL | 204114-003 | May 29, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for MEKINIST
When does loss-of-exclusivity occur for MEKINIST?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4102
Estimated Expiration: ⤷ Try a Trial
Patent: 2185
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 10306653
Estimated Expiration: ⤷ Try a Trial
Patent: 11349422
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2012008854
Estimated Expiration: ⤷ Try a Trial
Patent: 2013015602
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 75803
Estimated Expiration: ⤷ Try a Trial
Patent: 22701
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 12000964
Estimated Expiration: ⤷ Try a Trial
Patent: 13001779
Estimated Expiration: ⤷ Try a Trial
China
Patent: 2655753
Estimated Expiration: ⤷ Try a Trial
Patent: 3998041
Estimated Expiration: ⤷ Try a Trial
Colombia
Patent: 31498
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 120155
Estimated Expiration: ⤷ Try a Trial
Patent: 130352
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0191617
Estimated Expiration: ⤷ Try a Trial
Patent: 0201409
Estimated Expiration: ⤷ Try a Trial
Patent: 0221304
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 23376
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 88033
Estimated Expiration: ⤷ Try a Trial
Patent: 54736
Estimated Expiration: ⤷ Try a Trial
Patent: 60498
Estimated Expiration: ⤷ Try a Trial
Dominican Republic
Patent: 012000091
Estimated Expiration: ⤷ Try a Trial
Patent: 013000138
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 0589
Estimated Expiration: ⤷ Try a Trial
Patent: 5198
Estimated Expiration: ⤷ Try a Trial
Patent: 1290149
Estimated Expiration: ⤷ Try a Trial
Patent: 1390913
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 88033
Estimated Expiration: ⤷ Try a Trial
Patent: 54736
Estimated Expiration: ⤷ Try a Trial
Patent: 60498
Estimated Expiration: ⤷ Try a Trial
Patent: 08343
Estimated Expiration: ⤷ Try a Trial
Patent: 59204
Estimated Expiration: ⤷ Try a Trial
Patent: 59205
Estimated Expiration: ⤷ Try a Trial
Patent: 59217
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 46139
Estimated Expiration: ⤷ Try a Trial
Patent: 50788
Estimated Expiration: ⤷ Try a Trial
Patent: 60206
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 9073
Estimated Expiration: ⤷ Try a Trial
Patent: 6855
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 18929
Estimated Expiration: ⤷ Try a Trial
Patent: 26014
Estimated Expiration: ⤷ Try a Trial
Patent: 13508294
Estimated Expiration: ⤷ Try a Trial
Patent: 14510704
Estimated Expiration: ⤷ Try a Trial
Patent: 17137299
Estimated Expiration: ⤷ Try a Trial
Jordan
Patent: 94
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 88033
Estimated Expiration: ⤷ Try a Trial
Patent: 54736
Estimated Expiration: ⤷ Try a Trial
Patent: 60498
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 0501
Estimated Expiration: ⤷ Try a Trial
Patent: 4759
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 12004413
Estimated Expiration: ⤷ Try a Trial
Patent: 13007073
Estimated Expiration: ⤷ Try a Trial
Montenegro
Patent: 497
Estimated Expiration: ⤷ Try a Trial
Morocco
Patent: 746
Estimated Expiration: ⤷ Try a Trial
Patent: 883
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 8913
Estimated Expiration: ⤷ Try a Trial
Patent: 2157
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 121093
Estimated Expiration: ⤷ Try a Trial
Patent: 140040
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 88033
Estimated Expiration: ⤷ Try a Trial
Patent: 54736
Estimated Expiration: ⤷ Try a Trial
Patent: 60498
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 88033
Estimated Expiration: ⤷ Try a Trial
Patent: 54736
Estimated Expiration: ⤷ Try a Trial
Patent: 60498
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 181
Estimated Expiration: ⤷ Try a Trial
Patent: 702
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 1054
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 88033
Estimated Expiration: ⤷ Try a Trial
Patent: 54736
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1202612
Estimated Expiration: ⤷ Try a Trial
Patent: 1304189
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1729116
Estimated Expiration: ⤷ Try a Trial
Patent: 1911109
Estimated Expiration: ⤷ Try a Trial
Patent: 120104547
Estimated Expiration: ⤷ Try a Trial
Patent: 130130028
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 45479
Estimated Expiration: ⤷ Try a Trial
Patent: 20536
Estimated Expiration: ⤷ Try a Trial
Patent: 30157
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 05828
Estimated Expiration: ⤷ Try a Trial
Patent: 1249441
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 5064
Estimated Expiration: ⤷ Try a Trial
Patent: 3158
Estimated Expiration: ⤷ Try a Trial
Uruguay
Patent: 818
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering MEKINIST around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 3808343 | NOUVELLE COMPOSITION PHARMACEUTIQUE (NOVEL PHARMACEUTICAL COMPOSITION) | ⤷ Try a Trial |
| Poland | 2298768 | ⤷ Try a Trial | |
| Portugal | 2488033 | ⤷ Try a Trial | |
| European Patent Office | 4159217 | COMBINATION COMPRENANT UN INHIBITEUR DE MEK ET UN INHIBITEUR DE B-RAF (COMBINATION COMPRISING AN MEK INHIBITOR AND A B-RAF INHIBITOR) | ⤷ Try a Trial |
| Hungary | S1400055 | ⤷ Try a Trial | |
| Chile | 2013001779 | Comprimido farmaceutico que comprende el solvato de dimetilsulfoxido de n-{3-[3-ciclopropil-5-(2-fluoro-4-yodo-fenilamino)-6,8-dimetil-2,4,7-trioxo-3,4,6,7-tetrahidro-2h-pirido[4,3-d]pirimidin-1-il]fenil}acetamida; procedimiento de preparacion; y su uso para tratar el cancer. | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for MEKINIST
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1761528 | 68/2014 | Austria | ⤷ Try a Trial | PRODUCT NAME: TRAMETINIB, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH VERTRAEGLICHEN SALZES, HYDRATS ODER SOLVATS DAVON; REGISTRATION NO/DATE: EU/1/14/931 (MITTEILUNG) 20140702 |
| 1761528 | 205 5032-2014 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: TRAMETINIB, PRIPADNE VO FORME JEHO FARMACEUTICKY PRIJATELNEJ SOLI, HYDRATU ALEBO SOLVATU; REGISTRATION NO/DATE: EU/1/14/931 20140702 |
| 1761528 | C01761528/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: TRAMETINIB; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 65883 22.02.2016 |
| 1761528 | PA2014039 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: TRAMETINIBUM; REGISTRATION NO/DATE: EU/1/14/931/01-06 20140630 |
| 1761528 | C 2014 044 | Romania | ⤷ Try a Trial | PRODUCT NAME: TRAMETINIB, OPTIONAL SUB FORMA UNEI SARI, HIDRAT SAU SOLVAT AL ACESTUIA, ACCEPTABILE FARMACEUTIC; NATIONAL AUTHORISATION NUMBER: EU/1/14/931; DATE OF NATIONAL AUTHORISATION: 20140630; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/14/931; DATE OF FIRST AUTHORISATION IN EEA: 20140630 |
| 1761528 | 14C0083 | France | ⤷ Try a Trial | PRODUCT NAME: TRAMETINIB,EVENTUELLEMENT SOUS LA FORME D'UN SEL,HYDRATE OU SOLVATE PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; REGISTRATION NO/DATE: EU/1/14/931 20140630 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


