LEVAQUIN Drug Patent Profile
✉ Email this page to a colleague
When do Levaquin patents expire, and what generic alternatives are available?
Levaquin is a drug marketed by Janssen Pharms and is included in three NDAs.
The generic ingredient in LEVAQUIN is levofloxacin. There are thirty-one drug master file entries for this compound. Forty-four suppliers are listed for this compound. Additional details are available on the levofloxacin profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Levaquin
A generic version of LEVAQUIN was approved as levofloxacin by RISING on December 20th, 2010.
Summary for LEVAQUIN
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 98 |
| Clinical Trials: | 23 |
| Patent Applications: | 5,337 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for LEVAQUIN |
| What excipients (inactive ingredients) are in LEVAQUIN? | LEVAQUIN excipients list |
| DailyMed Link: | LEVAQUIN at DailyMed |

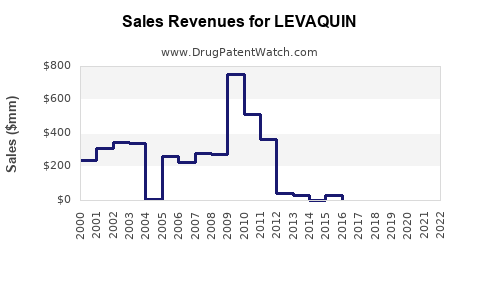
Recent Clinical Trials for LEVAQUIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Pfizer | Phase 2 |
| National Lung Hospital, Vietnam | Phase 2 |
| Boston University | Phase 2 |
Anatomical Therapeutic Chemical (ATC) Classes for LEVAQUIN
Paragraph IV (Patent) Challenges for LEVAQUIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| LEVAQUIN | Oral Solution | levofloxacin | 25 mg/mL | 021721 | 1 | 2009-07-30 |
US Patents and Regulatory Information for LEVAQUIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Pharms | LEVAQUIN | levofloxacin | INJECTABLE;INJECTION | 020635-001 | Dec 20, 1996 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen Pharms | LEVAQUIN IN DEXTROSE 5% IN PLASTIC CONTAINER | levofloxacin | INJECTABLE;INJECTION | 020635-002 | Dec 20, 1996 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen Pharms | LEVAQUIN | levofloxacin | TABLET;ORAL | 020634-001 | Dec 20, 1996 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for LEVAQUIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Pharms | LEVAQUIN | levofloxacin | INJECTABLE;INJECTION | 020635-004 | Dec 20, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| Janssen Pharms | LEVAQUIN | levofloxacin | SOLUTION;ORAL | 021721-001 | Oct 21, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| Janssen Pharms | LEVAQUIN | levofloxacin | SOLUTION;ORAL | 021721-001 | Oct 21, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for LEVAQUIN
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Chiesi Farmaceutici S.p.A | Quinsair | levofloxacin | EMEA/H/C/002789 Quinsair is indicated for the management of chronic pulmonary infections due to Pseudomonas aeruginosa in adult patients with cystic fibrosis.Consideration should be given to official guidance on the appropriate use of antibacterial agents. |
Authorised | no | no | no | 2015-03-25 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for LEVAQUIN
See the table below for patents covering LEVAQUIN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Yugoslavia | 74788 | ⤷ Try a Trial | |
| Croatia | P950176 | OPTICALLY ACTIVE BENZOXAZINE DERIVATIVES | ⤷ Try a Trial |
| Austria | 85057 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for LEVAQUIN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0206283 | SPC/GB97/085 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: LEVOFLOXACIN, OPTIONALLY IN THE FORM OF A HEMIHYDRATE; REGISTERED: UK 13402/0011 19970606; UK 13402/0012 19970606; UK 13402/0013 19970606 |
| 0206283 | C980016 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVOFLOXACINE, DESGEWENST IN DE VORM VAN EEN SOLVAAT, IN HET BIJZONDER LEVOFLOXACINE-HEMIHYDRAAT; NAT. REGISTRATION NO/DATE: RVG 21810 - RVG 21812 19971209; FIRST REGISTRATION: GB 13402/0011 - 13402/0013 19970606 |
| 0206283 | 98C0041 | Belgium | ⤷ Try a Trial | PRODUCT NAME: LEVOFLOXACINUM HEMIHYDRICUM; NAT. REGISTRATION NO/DATE: 354 IS 370 F3 19980624; FIRST REGISTRATION: GB 134020011 19970606 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


