LAMIVUDINE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Lamivudine, and when can generic versions of Lamivudine launch?
Lamivudine is a drug marketed by Aurobindo Pharma, Chartwell Molecular, Hetero Labs Ltd Iii, Annora, Apotex, Aurobindo Pharma Ltd, Aurobindo Pharma Usa, Breckenridge, Cipla, Hetero Labs Ltd V, Lupin Ltd, Macleods Pharms Ltd, Mylan, Mylan Labs Ltd, Strides Pharma, Upsher Smith Labs, Anda Repository, Chartwell Rx, Pharmacare, and Micro Labs. and is included in thirty-four NDAs.
The generic ingredient in LAMIVUDINE is lamivudine; nevirapine; zidovudine. There are twenty-nine drug master file entries for this compound. Additional details are available on the lamivudine; nevirapine; zidovudine profile page.
Summary for LAMIVUDINE
| US Patents: | 0 |
| Applicants: | 20 |
| NDAs: | 34 |
| Finished Product Suppliers / Packagers: | 13 |
| Raw Ingredient (Bulk) Api Vendors: | 146 |
| Clinical Trials: | 639 |
| Patent Applications: | 2,772 |
| Formulation / Manufacturing: | see details |
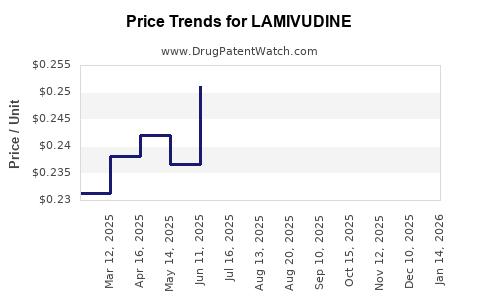
| Drug Prices: | Drug price information for LAMIVUDINE |
| What excipients (inactive ingredients) are in LAMIVUDINE? | LAMIVUDINE excipients list |
| DailyMed Link: | LAMIVUDINE at DailyMed |
Recent Clinical Trials for LAMIVUDINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| The Aurum Institute NPC | Phase 1/Phase 2 |
| Johns Hopkins University | Phase 1/Phase 2 |
| Centre de Recherches et d'Etude sur la Pathologie Tropicale et le Sida | N/A |
Pharmacology for LAMIVUDINE
Medical Subject Heading (MeSH) Categories for LAMIVUDINE
Anatomical Therapeutic Chemical (ATC) Classes for LAMIVUDINE
US Patents and Regulatory Information for LAMIVUDINE
EU/EMA Drug Approvals for LAMIVUDINE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Teva B.V. | Lamivudine Teva Pharma B.V. | lamivudine | EMEA/H/C/001111 Lamivudine Teva Pharma B.V. is indicated as part of antiretroviral combination therapy for the treatment of human-immunodeficiency-virus (HIV)-infected adults and children. |
Authorised | yes | no | no | 2009-12-10 | |
| Teva B.V. | Lamivudine Teva | lamivudine | EMEA/H/C/001113 Lamivudine Teva is indicated for the treatment of chronic hepatitis B in adults with:compensated liver disease with evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active liver inflammation and / or fibrosis. Initiation of lamivudine treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier is not available or appropriate (see in section 5.1). |
Authorised | yes | no | no | 2009-10-23 | |
| GlaxoSmithKline (Ireland) Limited | Zeffix | lamivudine | EMEA/H/C/000242 Zeffix is indicated for the treatment of chronic hepatitis B in adults with:, , , compensated liver disease with evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active liver inflammation and / or fibrosis. Initiation of lamivudine treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier is not available or appropriate;, decompensated liver disease in combination with a second agent without cross-resistance to lamivudine., , |
Authorised | no | no | no | 1999-07-29 | |
| ViiV Healthcare BV | Epivir | lamivudine | EMEA/H/C/000107 Epivir is indicated as part of antiretroviral combination therapy for the treatment of human-immunodeficiency-virus (HIV)-infected adults and children., |
Authorised | no | no | no | 1996-08-08 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |


