FLUPHENAZINE Drug Patent Profile
✉ Email this page to a colleague
When do Fluphenazine patents expire, and when can generic versions of Fluphenazine launch?
Fluphenazine is a drug marketed by Eugia Pharma, Fresenius Kabi Usa, Gland Pharma Ltd, Hikma, Hospira, MSN, Mylan Labs Ltd, Par Sterile Products, Teva Parenteral, Ani Pharms, Pharm Assoc, Ajanta Pharma Ltd, Amneal, Apotex, Aurobindo Pharma Ltd, Dr Reddys, Glenmark Pharms Ltd, Lannett Co Inc, Novitium Pharma, Prasco, Sandoz, Taro, Torrent, Twi Pharms, Upsher Smith Labs, Watson Labs, Zameer Pharms, and Zydus. and is included in thirty-six NDAs.
The generic ingredient in FLUPHENAZINE is fluphenazine hydrochloride. There are nineteen drug master file entries for this compound. Twenty-seven suppliers are listed for this compound. Additional details are available on the fluphenazine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Fluphenazine
A generic version of FLUPHENAZINE was approved as fluphenazine hydrochloride by FRESENIUS KABI USA on April 16th, 1987.
Summary for FLUPHENAZINE
| US Patents: | 0 |
| Applicants: | 28 |
| NDAs: | 36 |
| Formulation / Manufacturing: | see details |
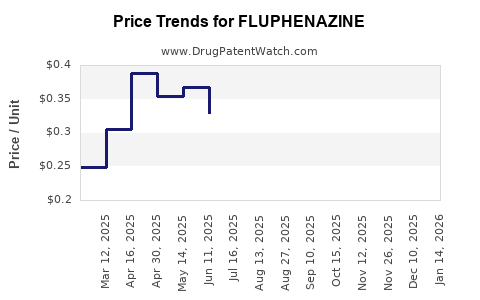
| Drug Prices: | Drug price information for FLUPHENAZINE |
| DailyMed Link: | FLUPHENAZINE at DailyMed |