ESZOPICLONE Drug Patent Profile
✉ Email this page to a colleague
When do Eszopiclone patents expire, and what generic alternatives are available?
Eszopiclone is a drug marketed by Aurobindo Pharma, Chartwell Rx, Dr Reddys, Glenmark Generics, Hetero Labs Ltd V, Hikma, Ipca Labs Ltd, Lupin Ltd, Macleods Pharms Ltd, Mylan Pharms Inc, Nostrum Labs Inc, Orbion Pharms, Sun Pharm, and Teva. and is included in fourteen NDAs.
The generic ingredient in ESZOPICLONE is eszopiclone. There are twenty drug master file entries for this compound. Thirty-two suppliers are listed for this compound. Additional details are available on the eszopiclone profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Eszopiclone
A generic version of ESZOPICLONE was approved as eszopiclone by TEVA on May 23rd, 2011.
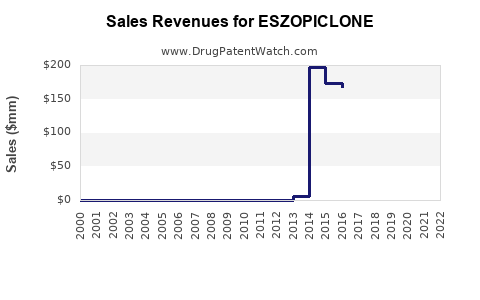
Summary for ESZOPICLONE
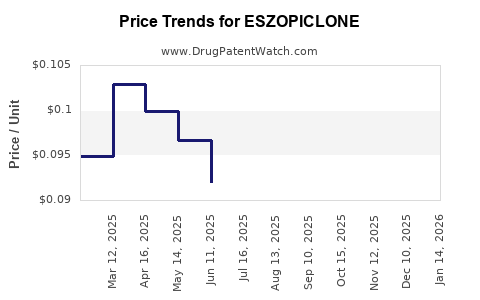
See drug prices for ESZOPICLONE
Recent Clinical Trials for ESZOPICLONE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Brigham and Women's Hospital | Phase 1/Phase 2 |
| Laboratorios Andromaco S.A. | Phase 1 |
| VA Office of Research and Development | Phase 3 |
Medical Subject Heading (MeSH) Categories for ESZOPICLONE
Anatomical Therapeutic Chemical (ATC) Classes for ESZOPICLONE
Paragraph IV (Patent) Challenges for ESZOPICLONE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| LUNESTA | Tablets | eszopiclone | 1 mg, 2 mg and 3 mg | 021476 | 10 | 2008-12-15 |
US Patents and Regulatory Information for ESZOPICLONE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva | ESZOPICLONE | eszopiclone | TABLET;ORAL | 091169-002 | May 23, 2011 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Nostrum Labs Inc | ESZOPICLONE | eszopiclone | TABLET;ORAL | 203087-002 | May 8, 2019 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mylan Pharms Inc | ESZOPICLONE | eszopiclone | TABLET;ORAL | 091151-001 | Mar 26, 2013 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Nostrum Labs Inc | ESZOPICLONE | eszopiclone | TABLET;ORAL | 203087-003 | May 8, 2019 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


