EMEND Drug Patent Profile
✉ Email this page to a colleague
When do Emend patents expire, and when can generic versions of Emend launch?
Emend is a drug marketed by Merck, Msd Merck Co, and Merck And Co Inc. and is included in three NDAs. There is one patent protecting this drug and three Paragraph IV challenges.
This drug has forty-eight patent family members in thirty-seven countries.
The generic ingredient in EMEND is fosaprepitant dimeglumine. There are eleven drug master file entries for this compound. Twenty-four suppliers are listed for this compound. Additional details are available on the fosaprepitant dimeglumine profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Emend
A generic version of EMEND was approved as fosaprepitant dimeglumine by FRESENIUS KABI USA on June 9th, 2016.
Summary for EMEND
| International Patents: | 48 |
| US Patents: | 1 |
| Applicants: | 3 |
| NDAs: | 3 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 93 |
| Patent Applications: | 2,784 |
| Formulation / Manufacturing: | see details |
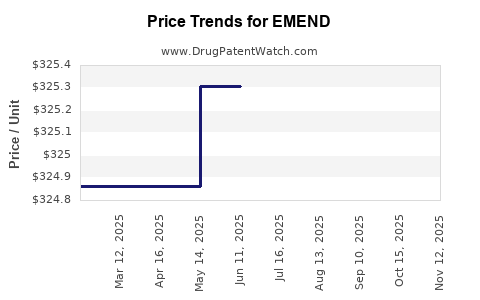
| Drug Prices: | Drug price information for EMEND |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for EMEND |
| What excipients (inactive ingredients) are in EMEND? | EMEND excipients list |
| DailyMed Link: | EMEND at DailyMed |
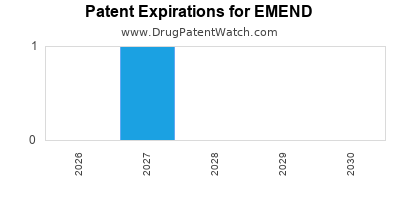
Paragraph IV (Patent) Challenges for EMEND
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| EMEND | for Oral Suspension | aprepitant | 125 mg/Kit | 207865 | 1 | 2016-11-23 |
| EMEND | Injection | fosaprepitant dimeglumine | 150 mg/vial | 022023 | 2 | 2012-01-25 |
| EMEND | Capsule | aprepitant | 40 mg, 80 mg and 125 mg | 021549 | 1 | 2008-11-03 |
US Patents and Regulatory Information for EMEND
EMEND is protected by three US patents and one FDA Regulatory Exclusivity.
Patents protecting EMEND
Pharmaceutical composition of a tachykinin receptor antagonist
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF POSTOPERATIVE NAUSEA AND VOMITING
Pharmaceutical composition of a tachykinin receptor antagonist
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: FOR THE PREVENTION OF NAUSEA AND VOMITING ASSOCIATED WITH CHEMOTHERAPY
Pharmaceutical composition of a tachykinin receptor antagonist
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF NAUSEA AND VOMITING ASSOCIATED WITH CHEMOTHERAPY (CINV)
FDA Regulatory Exclusivity protecting EMEND
ADDITION OF A 3-DAY FOSAPREPITANT FOR INJECTION INTRAVENOUS DOSING REGIMEN IN PEDIATRIC PATIENTS FOR THE CURRENTLY APPROVED PREVENTION OF CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Merck | EMEND | aprepitant | CAPSULE;ORAL | 021549-003 | Jun 30, 2006 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Msd Merck Co | EMEND | aprepitant | FOR SUSPENSION;ORAL | 207865-001 | Dec 17, 2015 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Merck And Co Inc | EMEND | fosaprepitant dimeglumine | POWDER;INTRAVENOUS | 022023-001 | Jan 25, 2008 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Merck | EMEND | aprepitant | CAPSULE;ORAL | 021549-001 | Mar 26, 2003 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Merck | EMEND | aprepitant | CAPSULE;ORAL | 021549-002 | Mar 26, 2003 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Merck And Co Inc | EMEND | fosaprepitant dimeglumine | POWDER;INTRAVENOUS | 022023-002 | Nov 12, 2010 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for EMEND
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Merck And Co Inc | EMEND | fosaprepitant dimeglumine | POWDER;INTRAVENOUS | 022023-002 | Nov 12, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Merck | EMEND | aprepitant | CAPSULE;ORAL | 021549-003 | Jun 30, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Merck And Co Inc | EMEND | fosaprepitant dimeglumine | POWDER;INTRAVENOUS | 022023-002 | Nov 12, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Merck | EMEND | aprepitant | CAPSULE;ORAL | 021549-003 | Jun 30, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Merck | EMEND | aprepitant | CAPSULE;ORAL | 021549-001 | Mar 26, 2003 | ⤷ Try a Trial | ⤷ Try a Trial |
| Merck | EMEND | aprepitant | CAPSULE;ORAL | 021549-003 | Jun 30, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Merck | EMEND | aprepitant | CAPSULE;ORAL | 021549-001 | Mar 26, 2003 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for EMEND
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Merck Sharp & Dohme B.V. | Emend | aprepitant | EMEA/H/C/000527 Emend 40 mg hard capsules is indicated for the prevention of postoperative nausea and vomiting (PONV) in adults.Emend is also available as 80 mg and 125 mg hard capsules for the prevention of nausea and vomiting associated with highly and moderately emetogenic cancer chemotherapy in adults and adolescents from the age of 12 (see separate Summary of Product Characteristics).Emend is also available as 165 mg hard capsules for the prevention of acute and delayed nausea and vomiting associated with highly emetogenic cisplatin based cancer chemotherapy in adults and the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy in adults.Emend is also available as powder for oral suspension for the prevention of nausea and vomiting associated with highly and moderately emetogenic cancer chemotherapy in children, toddlers and infants from the age of 6 months to less than 12 years.Emend 80 mg, 125 mg, 165 mg hard capsules and Emend powder for oral suspension are given as part of combination therapy. |
Authorised | no | no | no | 2003-11-11 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for EMEND
See the table below for patents covering EMEND around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Norway | 310074 | ⤷ Try a Trial | |
| Norway | 932403 | ⤷ Try a Trial | |
| Austria | 195416 | ⤷ Try a Trial | |
| European Patent Office | 0577394 | Morpholine et thiomorpholine, antagonistes du récepteur de tachykinine (Morpholine and thiomorpholine tachykinin receptor antagonists) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 9901444 | ⤷ Try a Trial | |
| Hungary | 228003 | POLYMORPHIC FORM OF A MORPHOLINE DERIVATIVE HAVING TACHYKININ RECEPTOR ANTAGONIST ACTIVITY AND PHARMACEUTICAL COMPOSITIONS CONTAINING THE SAME | ⤷ Try a Trial |
| Georgia, Republic of | P20063875 | PHARMACEUTICAL NANOPARTICULATE COMPOSITION OF A TACHYKININ RECEPTOR ANTAGONIST | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for EMEND
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0734381 | SPC/GB04/011 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: APREPITANT, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT; REGISTERED: UK EU/1/03/262/001 20031113; UK EU/1/03/262/002 20031113; UK EU/1/03/262/003 20031113; UK EU/1/03/262/004 20031113; UK EU/1/03/262/005 20031113; UK EU/1/03/262/006 20031113 |
| 0748320 | 08C0019 | France | ⤷ Try a Trial | PRODUCT NAME: FOSAPREPITANT DIMEGLUMINE; REGISTRATION NO/DATE: EU/1/07/437/001 20080111 |
| 0734381 | SPC005/2004 | Ireland | ⤷ Try a Trial | SPC005/2004: 20050504, EXPIRES: 20181110 |
| 0734381 | 3/2004 | Austria | ⤷ Try a Trial | PRODUCT NAME: APREPITANT, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEMBAREN SALZES; REGISTRATION NO/DATE: EU/1/03/262/001 - EU/1/03/262/006 20031111 |
| 0734381 | 300146 | Netherlands | ⤷ Try a Trial | 300146, 20141213, EXPIRES: 20181110 |
| 0734381 | PA2004002 | Lithuania | ⤷ Try a Trial | PRODCUT NAME: 5-[[(2R,3S)-2-[(1R)-1-[3,5-BIS(TRIFLUORMETIL)FENIL]ETOKSI]-3-(4-FLUORFENIL)-4-MORFOLINIL]METIL]-1,2-DIHIDRO-3H-1,2,4-TRIAZOL-3-ONAS (APREPITANTAS); REGISTRATION NO./DATE: EU/1/03/262/001-006/20031111 |
| 0734381 | CA 2004 00009 | Denmark | ⤷ Try a Trial | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


