CHILDREN Drug Patent Profile
✉ Email this page to a colleague
When do Children patents expire, and when can generic versions of Children launch?
Children is a drug marketed by Haleon Us Holdings, Bausch And Lomb, Chattem Sanofi, Bayer Hlthcare, Amneal Pharms, Apozeal Pharms, Aurobindo Pharma, Bajaj, Chartwell Molecular, Chartwell Rx, Cypress Pharm, Hetero Labs Ltd Iii, Perrigo R And D, Pharm Assoc, Quagen, Ranbaxy Labs Ltd, Taro, Tris Pharma Inc, Jubilant Generics, Novel Labs Inc, Sandoz, Sun Pharm, Bayer Healthcare Llc, Moberg Pharma North, P And L, Dr Reddys Labs Ltd, Aurobindo Pharma Ltd, Hetero Labs Ltd V, Rising, Sun Pharm Inds, Teva, Wockhardt, Perrigo, and J And J Consumer Inc. and is included in forty-nine NDAs. There are five patents protecting this drug.
The generic ingredient in CHILDREN is loratadine. There are thirty-nine drug master file entries for this compound. One hundred and fifty suppliers are listed for this compound. Additional details are available on the loratadine profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Children
A generic version of CHILDREN was approved as loratadine by PLD ACQUISITIONS LLC on January 21st, 2003.
Summary for CHILDREN
| US Patents: | 0 |
| Applicants: | 34 |
| NDAs: | 49 |
| Formulation / Manufacturing: | see details |
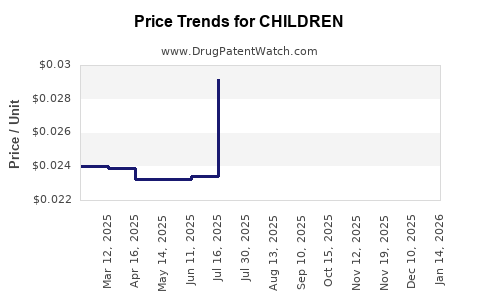
| Drug Prices: | Drug price information for CHILDREN |
| DailyMed Link: | CHILDREN at DailyMed |