BENZTROPINE MESYLATE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Benztropine Mesylate, and when can generic versions of Benztropine Mesylate launch?
Benztropine Mesylate is a drug marketed by Fresenius Kabi Usa, Hikma, Hikma Farmaceutica, Luitpold, Navinta Llc, Aiping Pharm Inc, Aspen Global Inc, Chartwell Rx, Epic Pharma Llc, Invagen Pharms, Lannett Co Inc, Leading, Oxford Pharms, Pliva, Quantum Pharmics, Usl Pharma, and Vintage. and is included in twenty-seven NDAs.
The generic ingredient in BENZTROPINE MESYLATE is benztropine mesylate. There are seven drug master file entries for this compound. Eighteen suppliers are listed for this compound. Additional details are available on the benztropine mesylate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Benztropine Mesylate
A generic version of BENZTROPINE MESYLATE was approved as benztropine mesylate by PLIVA on August 10th, 1988.
Summary for BENZTROPINE MESYLATE
| US Patents: | 0 |
| Applicants: | 17 |
| NDAs: | 27 |
| Finished Product Suppliers / Packagers: | 18 |
| Raw Ingredient (Bulk) Api Vendors: | 124 |
| Clinical Trials: | 1 |
| Patent Applications: | 1,303 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for BENZTROPINE MESYLATE |
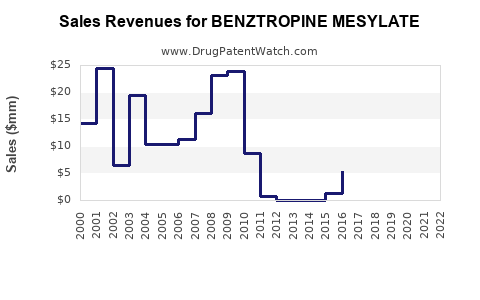
| Drug Sales Revenues: | Drug sales revenues for BENZTROPINE MESYLATE |
| What excipients (inactive ingredients) are in BENZTROPINE MESYLATE? | BENZTROPINE MESYLATE excipients list |
| DailyMed Link: | BENZTROPINE MESYLATE at DailyMed |

See drug prices for BENZTROPINE MESYLATE
Recent Clinical Trials for BENZTROPINE MESYLATE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Institute of Allergy and Infectious Diseases (NIAID) | Phase 2 |
| Boehringer Ingelheim | Phase 2 |
Pharmacology for BENZTROPINE MESYLATE
| Drug Class | Anticholinergic Antihistamine |
| Mechanism of Action | Cholinergic Antagonists Histamine Receptor Antagonists |


