BRILINTA Drug Patent Profile
✉ Email this page to a colleague
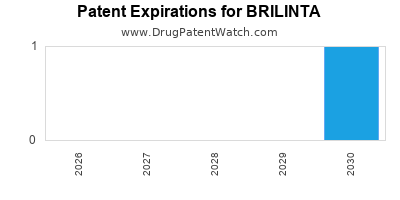
When do Brilinta patents expire, and when can generic versions of Brilinta launch?
Brilinta is a drug marketed by Astrazeneca and is included in one NDA. There are three patents protecting this drug and two Paragraph IV challenges.
This drug has one hundred and forty-six patent family members in forty-four countries.
The generic ingredient in BRILINTA is ticagrelor. There are twenty-one drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the ticagrelor profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Brilinta
A generic version of BRILINTA was approved as ticagrelor by HISUN PHARM HANGZHOU on January 23rd, 2019.
Summary for BRILINTA
| International Patents: | 146 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 101 |
| Clinical Trials: | 89 |
| Patent Applications: | 1,009 |
| Formulation / Manufacturing: | see details |
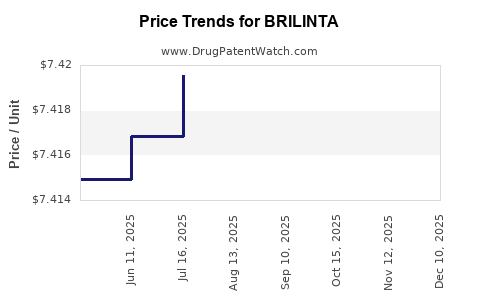
| Drug Prices: | Drug price information for BRILINTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BRILINTA |
| What excipients (inactive ingredients) are in BRILINTA? | BRILINTA excipients list |
| DailyMed Link: | BRILINTA at DailyMed |
Recent Clinical Trials for BRILINTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Yonsei University | Phase 4 |
| University of Florida | Phase 2/Phase 3 |
| Medical University of South Carolina | Phase 2/Phase 3 |
Pharmacology for BRILINTA
| Drug Class | P2Y12 Platelet Inhibitor |
| Mechanism of Action | Cytochrome P450 3A4 Inhibitors P-Glycoprotein Inhibitors P2Y12 Receptor Antagonists |
| Physiological Effect | Decreased Platelet Aggregation |
Anatomical Therapeutic Chemical (ATC) Classes for BRILINTA
Paragraph IV (Patent) Challenges for BRILINTA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BRILINTA | Tablets | ticagrelor | 60 mg | 022433 | 3 | 2015-09-30 |
| BRILINTA | Tablets | ticagrelor | 90 mg | 022433 | 16 | 2015-07-20 |
US Patents and Regulatory Information for BRILINTA
BRILINTA is protected by three US patents and four FDA Regulatory Exclusivities.
Patents protecting BRILINTA
Method of treating or prevention of atherothrombotic events in patients with history of myocardial infarction
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Triazolo(4,5-D)pyrimidine compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting BRILINTA
REDUCE THE RISK OF STROKE IN PATIENTS WITH ACUTE ISCHEMIC STROKE (NIH STROKE SCALE SCORE
Exclusivity Expiration: ⤷ Try a Trial
INFORMATION ADDED TO SECTION 8.4 OF THE LABELING TO INCLUDE THE RESULT OF STUDY HESTIA3
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca | BRILINTA | ticagrelor | TABLET;ORAL | 022433-002 | Sep 3, 2015 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca | BRILINTA | ticagrelor | TABLET;ORAL | 022433-002 | Sep 3, 2015 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Astrazeneca | BRILINTA | ticagrelor | TABLET;ORAL | 022433-002 | Sep 3, 2015 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BRILINTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca | BRILINTA | ticagrelor | TABLET;ORAL | 022433-002 | Sep 3, 2015 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca | BRILINTA | ticagrelor | TABLET;ORAL | 022433-002 | Sep 3, 2015 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca | BRILINTA | ticagrelor | TABLET;ORAL | 022433-002 | Sep 3, 2015 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for BRILINTA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AstraZeneca AB | Brilique | ticagrelor | EMEA/H/C/001241 Brilique, co administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients withacute coronary syndromes (ACS) ora history of myocardial infarction (MI) and a high risk of developing an atherothrombotic eventBrilique, co-administered with acetyl salicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with a history of myocardial infarction (MI occurred at least one year ago) and a high risk of developing an atherothrombotic event. |
Authorised | no | no | no | 2010-12-03 | |
| AstraZeneca AB | Possia | ticagrelor | EMEA/H/C/002303 Possia, co-administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with acute coronary syndromes (unstable angina, non-ST-elevation myocardial infarction [NSTEMI] or ST-elevation myocardial infarction [STEMI]); including patients managed medically, and those who are managed with percutaneous coronary intervention (PCI) or coronary artery by-pass grafting (CABG). |
Withdrawn | no | no | no | 2010-12-03 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for BRILINTA
When does loss-of-exclusivity occur for BRILINTA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2451
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 07288541
Estimated Expiration: ⤷ Try a Trial
Patent: 11205164
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0715712
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 59328
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 07002421
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1505754
Estimated Expiration: ⤷ Try a Trial
Colombia
Patent: 50163
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0170694
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 19380
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 56832
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 56832
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 31939
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 6700
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 85139
Estimated Expiration: ⤷ Try a Trial
Patent: 10501554
Estimated Expiration: ⤷ Try a Trial
Patent: 14040448
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 56832
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 7966
Estimated Expiration: ⤷ Try a Trial
Patent: 5009
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 0403
Estimated Expiration: ⤷ Try a Trial
Patent: 09001853
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 4514
Estimated Expiration: ⤷ Try a Trial
Patent: 6700
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 1787
Estimated Expiration: ⤷ Try a Trial
Patent: 090425
Estimated Expiration: ⤷ Try a Trial
Philippines
Patent: 013501627
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 56832
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 56832
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 76223
Estimated Expiration: ⤷ Try a Trial
Patent: 09104330
Estimated Expiration: ⤷ Try a Trial
Patent: 12153069
Estimated Expiration: ⤷ Try a Trial
Saudi Arabia
Patent: 280442
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 884
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 7162
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 56832
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0900991
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1539467
Estimated Expiration: ⤷ Try a Trial
Patent: 090055561
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 25930
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 82772
Estimated Expiration: ⤷ Try a Trial
Patent: 0817412
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 105
Estimated Expiration: ⤷ Try a Trial
Uruguay
Patent: 551
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BRILINTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 4202607 | ⤷ Try a Trial | |
| Poland | 392882 | ⤷ Try a Trial | |
| Norway | 333289 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BRILINTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1135391 | SPC/GB11/016 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TICAGRELOR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF.; REGISTERED: UK EU/1/10/655/001 20101203; UK EU/1/10/655/002 20101203; UK EU/1/10/655/003 20101203; UK EU/1/10/655/004 20101203; UK EU/1/10/655/005 20101203; UK EU/1/10/655/006 20101203 |
| 1135391 | C01135391/01 | Switzerland | ⤷ Try a Trial | FORMER REPRESENTATIVE: BOHEST AG, CH |
| 1135391 | 406 | Finland | ⤷ Try a Trial | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


