VALCHLOR Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Valchlor, and what generic alternatives are available?
Valchlor is a drug marketed by Helsinn and is included in one NDA. There are six patents protecting this drug.
This drug has fifty patent family members in twenty countries.
The generic ingredient in VALCHLOR is mechlorethamine hydrochloride. There is one drug master file entry for this compound. One supplier is listed for this compound. Additional details are available on the mechlorethamine hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Valchlor
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 7, 2026. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for VALCHLOR
| International Patents: | 50 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 34 |
| Clinical Trials: | 7 |
| Patent Applications: | 4,961 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for VALCHLOR |
| What excipients (inactive ingredients) are in VALCHLOR? | VALCHLOR excipients list |
| DailyMed Link: | VALCHLOR at DailyMed |
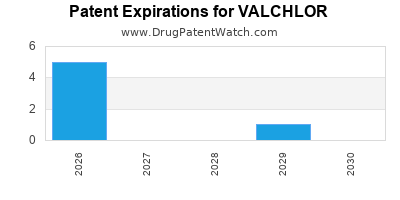

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VALCHLOR
Generic Entry Date for VALCHLOR*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
GEL;TOPICAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VALCHLOR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Centre for Human Drug Research, Netherlands | N/A |
| Recordati Rare Diseases | N/A |
| Sidney Kimmel Cancer Center at Thomas Jefferson University | Phase 2 |
Pharmacology for VALCHLOR
| Drug Class | Alkylating Drug |
| Mechanism of Action | Alkylating Activity |
US Patents and Regulatory Information for VALCHLOR
VALCHLOR is protected by six US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VALCHLOR is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting VALCHLOR
Stabilized compositions of volatile alkylating agents and methods of using thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Stabilized compositions of volatile alkylating agents and methods of using thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ALKYLATING DRUG INDICATED FOR THE TOPICAL TREATMENT OF STAGE IA AND IB MYCOSIS FUNGOIDES-TYPE CUTANEOUS T-CELL LYMPHOMA IN PATIENTS WHO HAVE RECEIVED PRIOR SKIN DIRECTED THERAPY
Stabilized compositions of volatile alkylating agents and methods of using thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Stabilized compositions of alkylating agents and methods of using same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Stabilized compositions of volatile alkylating agents and methods of using thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ALKYLATING DRUG INDICATED FOR THE TOPICAL TREATMENT OF STAGE IA AND IB MYCOSIS FUNGOIDES-TYPE CUTANEOUS T-CELL LYMPHOMA IN PATIENTS WHO HAVE RECEIVED PRIOR SKIN DIRECTED THERAPY
Stabilized compositions of volatile alkylating agents and methods of using thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Helsinn | VALCHLOR | mechlorethamine hydrochloride | GEL;TOPICAL | 202317-001 | Aug 23, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Helsinn | VALCHLOR | mechlorethamine hydrochloride | GEL;TOPICAL | 202317-001 | Aug 23, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Helsinn | VALCHLOR | mechlorethamine hydrochloride | GEL;TOPICAL | 202317-001 | Aug 23, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Helsinn | VALCHLOR | mechlorethamine hydrochloride | GEL;TOPICAL | 202317-001 | Aug 23, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Helsinn | VALCHLOR | mechlorethamine hydrochloride | GEL;TOPICAL | 202317-001 | Aug 23, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Helsinn | VALCHLOR | mechlorethamine hydrochloride | GEL;TOPICAL | 202317-001 | Aug 23, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for VALCHLOR
When does loss-of-exclusivity occur for VALCHLOR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 06223076
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 00468
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1175735
Estimated Expiration: ⤷ Try a Trial
Patent: 2036557
Estimated Expiration: ⤷ Try a Trial
Patent: 7149591
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0190551
Estimated Expiration: ⤷ Try a Trial
Patent: 0210283
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 13305
Estimated Expiration: ⤷ Try a Trial
Patent: 21777
Estimated Expiration: ⤷ Try a Trial
Patent: 23974
Estimated Expiration: ⤷ Try a Trial
Patent: 17027
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 58864
Estimated Expiration: ⤷ Try a Trial
Patent: 73876
Estimated Expiration: ⤷ Try a Trial
Patent: 94960
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 58864
Estimated Expiration: ⤷ Try a Trial
Patent: 73876
Estimated Expiration: ⤷ Try a Trial
Patent: 94960
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 19675
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 43767
Estimated Expiration: ⤷ Try a Trial
Patent: 53482
Estimated Expiration: ⤷ Try a Trial
Patent: 700033
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 36457
Estimated Expiration: ⤷ Try a Trial
Patent: 71514
Estimated Expiration: ⤷ Try a Trial
Patent: 08533152
Estimated Expiration: ⤷ Try a Trial
Patent: 13091643
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 858864
Estimated Expiration: ⤷ Try a Trial
Patent: 2017026
Estimated Expiration: ⤷ Try a Trial
Patent: 73876
Estimated Expiration: ⤷ Try a Trial
Patent: 94960
Estimated Expiration: ⤷ Try a Trial
Luxembourg
Patent: 0033
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 1268
Estimated Expiration: ⤷ Try a Trial
Patent: 9188
Estimated Expiration: ⤷ Try a Trial
Patent: 9001
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 58864
Estimated Expiration: ⤷ Try a Trial
Patent: 73876
Estimated Expiration: ⤷ Try a Trial
Patent: 94960
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 58864
Estimated Expiration: ⤷ Try a Trial
Patent: 73876
Estimated Expiration: ⤷ Try a Trial
Patent: 94960
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 58864
Estimated Expiration: ⤷ Try a Trial
Patent: 73876
Estimated Expiration: ⤷ Try a Trial
Patent: 94960
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1168252
Estimated Expiration: ⤷ Try a Trial
Patent: 070120163
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 88744
Estimated Expiration: ⤷ Try a Trial
Patent: 27523
Estimated Expiration: ⤷ Try a Trial
Patent: 48841
Estimated Expiration: ⤷ Try a Trial
Turkey
Patent: 1907794
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VALCHLOR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| New Zealand | 599001 | compositions comprising bis(2-chloroethyl)methylamine (mechlorethamine) | ⤷ Try a Trial |
| Spain | 2388744 | ⤷ Try a Trial | |
| Denmark | 3494960 | ⤷ Try a Trial | |
| Turkey | 201907794 | ⤷ Try a Trial | |
| Lithuania | 3494960 | ⤷ Try a Trial | |
| China | 101175735 | Stabilized compositions of volatile alkylating agents and methods of using thereof | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VALCHLOR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1858864 | 36/2017 | Austria | ⤷ Try a Trial | PRODUCT NAME: CHLORMETHIN; REGISTRATION NO/DATE: EU/1/16/1171 (MITTEILUNG) 20170307 |
| 1858864 | 122017000059 | Germany | ⤷ Try a Trial | PRODUCT NAME: CHLORMETHIN; REGISTRATION NO/DATE: EU/1/16/1171 20170303 |
| 1858864 | C20170027 00259 | Estonia | ⤷ Try a Trial | PRODUCT NAME: KLOORMETIIN;REG NO/DATE: EU/1/16/1171 07.03.2017 |
| 1858864 | LUC00033 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: CHLORMETHINE ET SES DERIVES PHARMACEUTIQUEMENT ACCEPTABLES (LEDAGA); AUTHORISATION NUMBER AND DATE: EU/1/1671171 20170307 |
| 1858864 | 132017000093882 | Italy | ⤷ Try a Trial | PRODUCT NAME: CLORMETINA(LEDAGA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/16/1171, 20170307 |
| 1858864 | 2017C/033 | Belgium | ⤷ Try a Trial | DETAILS ASSIGNMENT: CHANGE OF OWNER(S), CESSION |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


