SYMBICORT Drug Patent Profile
✉ Email this page to a colleague
When do Symbicort patents expire, and what generic alternatives are available?
Symbicort is a drug marketed by Astrazeneca and is included in two NDAs. There are seven patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and sixty-two patent family members in thirty-nine countries.
The generic ingredient in SYMBICORT is budesonide; formoterol fumarate. There are twenty-two drug master file entries for this compound. Additional details are available on the budesonide; formoterol fumarate profile page.
DrugPatentWatch® Generic Entry Outlook for Symbicort
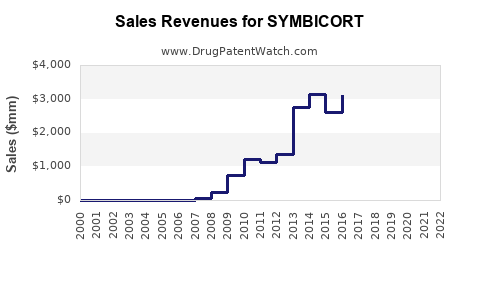
Annual sales in 2021 were $4.6bn, indicating a strong incentive for generic entry (peak sales were $5.7bn in 2019).
There have been eleven patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for SYMBICORT
| International Patents: | 162 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 3 |
| Clinical Trials: | 143 |
| Formulation / Manufacturing: | see details |
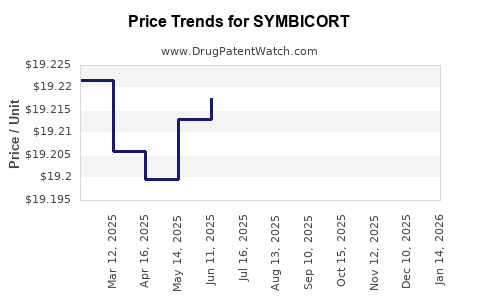
| Drug Prices: | Drug price information for SYMBICORT |
| Drug Sales Revenues: | Drug sales revenues for SYMBICORT |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for SYMBICORT |
| What excipients (inactive ingredients) are in SYMBICORT? | SYMBICORT excipients list |
| DailyMed Link: | SYMBICORT at DailyMed |
Recent Clinical Trials for SYMBICORT
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Cipla Ltd. | Phase 3 |
| Assistance Publique - Hôpitaux de Paris | Phase 3 |
| Istituto per la Ricerca e l'Innovazione Biomedica | Phase 4 |
Pharmacology for SYMBICORT
| Drug Class | Corticosteroid beta2-Adrenergic Agonist |
| Mechanism of Action | Adrenergic beta2-Agonists Corticosteroid Hormone Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for SYMBICORT
Paragraph IV (Patent) Challenges for SYMBICORT
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SYMBICORT | Metered Inhalation | budesonide; formoterol fumarate dihydrate | 80 mcg/4.5 mcg per inhalation and 160 mcg/4.5 mcg per inhalation | 021929 | 1 | 2018-06-26 |
US Patents and Regulatory Information for SYMBICORT
SYMBICORT is protected by five US patents.
Patents protecting SYMBICORT
Inhaler device counter
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Inhaler cap strap
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Inhaler device that reduces the risk for miscounting a dosage
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Inhalation device and a method for assembling said inhalation device
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Inhaler cap strap
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca | SYMBICORT | budesonide; formoterol fumarate dihydrate | AEROSOL, METERED;INHALATION | 021929-001 | Jul 21, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Astrazeneca | SYMBICORT | budesonide; formoterol fumarate dihydrate | AEROSOL, METERED;INHALATION | 021929-002 | Jul 21, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Astrazeneca | SYMBICORT | budesonide; formoterol fumarate dihydrate | AEROSOL, METERED;INHALATION | 021929-001 | Jul 21, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Astrazeneca | SYMBICORT AEROSPHERE | budesonide; formoterol fumarate | AEROSOL, METERED;INHALATION | 216579-001 | Apr 28, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Astrazeneca | SYMBICORT | budesonide; formoterol fumarate dihydrate | AEROSOL, METERED;INHALATION | 021929-002 | Jul 21, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SYMBICORT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca | SYMBICORT | budesonide; formoterol fumarate dihydrate | AEROSOL, METERED;INHALATION | 021929-002 | Jul 21, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca | SYMBICORT | budesonide; formoterol fumarate dihydrate | AEROSOL, METERED;INHALATION | 021929-002 | Jul 21, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca | SYMBICORT | budesonide; formoterol fumarate dihydrate | AEROSOL, METERED;INHALATION | 021929-001 | Jul 21, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca | SYMBICORT | budesonide; formoterol fumarate dihydrate | AEROSOL, METERED;INHALATION | 021929-001 | Jul 21, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca | SYMBICORT | budesonide; formoterol fumarate dihydrate | AEROSOL, METERED;INHALATION | 021929-001 | Jul 21, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for SYMBICORT
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Teva Pharma B.V. | Budesonide/Formoterol Teva Pharma B.V. | budesonide, formoterol fumarate dihydrate | EMEA/H/C/004882 Budesonide/Formoterol Teva Pharma B.V. is indicated in adults 18 years of age and older only.AsthmaBudesonide/Formoterol Teva Pharma B.V. is indicated in the regular treatment of asthma, where use of a combination (inhaled corticosteroid and long-acting β2 adrenoceptor agonist) is appropriate:-in patients not adequately controlled with inhaled corticosteroids and “as needed” inhaled short-acting β2 adrenoceptor agonists.or-in patients already adequately controlled on both inhaled corticosteroids and long-acting β2 adrenoceptor agonists.COPDSymptomatic treatment of patients with COPD with forced expiratory volume in 1 second (FEV1) |
Authorised | no | no | no | 2020-04-03 | |
| Teva Pharma B.V. | Budesonide/Formoterol Teva Pharma B.V. | budesonide, formoterol fumarate dihydrate | EMEA/H/C/003953 Budesonide/Formoterol Teva Pharma B.V. is indicated in adults 18 years of age and older only.AsthmaBudesonide/Formoterol Teva Pharma B.V. is indicated in the regular treatment of asthma, where use of a combination (inhaled corticosteroid and long-acting β2 adrenoceptor agonist) is appropriate: orin patients not adequately controlled with inhaled corticosteroids and “as needed” inhaled short-acting β2 adrenoceptor agonists.in patients already adequately controlled on both inhaled corticosteroids and long-acting β2 adrenoceptor agonists. |
Withdrawn | no | no | no | 2014-11-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for SYMBICORT
See the table below for patents covering SYMBICORT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Spain | 2289574 | ⤷ Try a Trial | |
| Israel | 134773 | USE FOR BUDESONIDE AND FORMOTEROL | ⤷ Try a Trial |
| Japan | 4282229 | ⤷ Try a Trial | |
| Mexico | 9205483 | COMPOSICIONES DE AEROSOL A PRESION | ⤷ Try a Trial |
| Norway | 20001401 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SYMBICORT
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0613371 | 2002C/022 | Belgium | ⤷ Try a Trial | PRODUCT NAME: BUDESONID. MICRONIS. AND FORMOTEROL. FUMARAS DIHYDR., NATL REGISTRATION NO/DATE: 624 IS 234 F 0 20010129; FIRST REGISTRATION: SE 16047 20000825 |
| 0613371 | SPC/GB02/033 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: FORMOTEROL (OPTIONALLY IN THE FORM OF THE FREE BASE OR A PHYSIOLOGICALLY ACCEPTABLE SALT THEREOF, OR A SOLVATE OF SUCH FREE BASE OR SALT ESPECIALLY AS FORMOTEROL FUMARATE DIHYDRATE) AND BUDESONIDE; REGISTERED: SE SE16047, 16048 20000825; UK PL17901/0091 20010515; UK PL17901/0092 20010515 |
| 0613371 | SPC016/2002 | Ireland | ⤷ Try a Trial | SPC016/2002: 20041105, EXPIRES: 20150824 |
| 0613371 | CA 2002 00019 | Denmark | ⤷ Try a Trial | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


