MYRBETRIQ Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Myrbetriq, and when can generic versions of Myrbetriq launch?
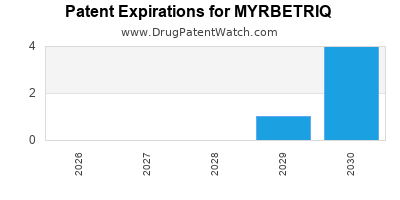
Myrbetriq is a drug marketed by Apgdi and is included in two NDAs. There are eight patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and eleven patent family members in thirty countries.
The generic ingredient in MYRBETRIQ is mirabegron. There are nineteen drug master file entries for this compound. Six suppliers are listed for this compound. Additional details are available on the mirabegron profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Myrbetriq
A generic version of MYRBETRIQ was approved as mirabegron by LUPIN LTD on September 28th, 2022.
Summary for MYRBETRIQ
| International Patents: | 111 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 74 |
| Clinical Trials: | 48 |
| Patent Applications: | 57 |
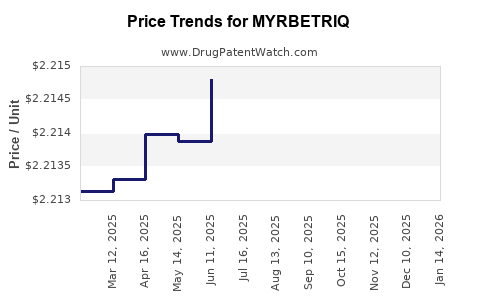
| Drug Prices: | Drug price information for MYRBETRIQ |
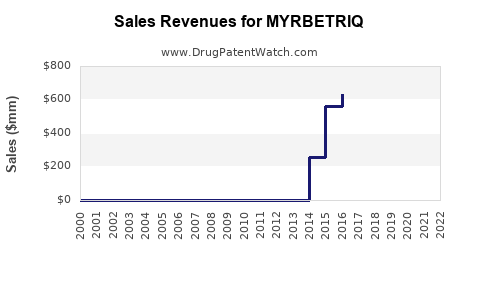
| Drug Sales Revenues: | Drug sales revenues for MYRBETRIQ |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for MYRBETRIQ |
| What excipients (inactive ingredients) are in MYRBETRIQ? | MYRBETRIQ excipients list |
| DailyMed Link: | MYRBETRIQ at DailyMed |
Recent Clinical Trials for MYRBETRIQ
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| The University of Texas Medical Branch, Galveston | Early Phase 1 |
| University of California, San Diego | Phase 1 |
| Shachi Tyagi | Phase 4 |
Pharmacology for MYRBETRIQ
Anatomical Therapeutic Chemical (ATC) Classes for MYRBETRIQ
Paragraph IV (Patent) Challenges for MYRBETRIQ
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| MYRBETRIQ | Extended-release Tablets | mirabegron | 50 mg | 202611 | 6 | 2016-06-28 |
US Patents and Regulatory Information for MYRBETRIQ
MYRBETRIQ is protected by seven US patents and two FDA Regulatory Exclusivities.
Patents protecting MYRBETRIQ
Pharmaceutical composition for modified release
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
.alpha.-form or .beta.-form crystal of acetanilide derivative
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
.alpha.-form or .beta.-form crystal of acetanilide derivative
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical composition for treating overactive bladder
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Remedy for overactive bladder comprising acetic acid anilide derivative as the active ingredient
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Remedy for overactive bladder comprising acetic acid anilide derivative as the active ingredient
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting MYRBETRIQ
TREATMENT OF NEUROGENIC DETRUSOR OVERACTIVITY (NDO) IN PEDIATRIC PATIENTS 3 YEARS AND OLDER AND WEIGHING 35 KILOGRAMS OR MORE
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apgdi | MYRBETRIQ | mirabegron | TABLET, EXTENDED RELEASE;ORAL | 202611-001 | Jun 28, 2012 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Apgdi | MYRBETRIQ | mirabegron | TABLET, EXTENDED RELEASE;ORAL | 202611-002 | Jun 28, 2012 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Apgdi | MYRBETRIQ | mirabegron | TABLET, EXTENDED RELEASE;ORAL | 202611-001 | Jun 28, 2012 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Apgdi | MYRBETRIQ | mirabegron | TABLET, EXTENDED RELEASE;ORAL | 202611-001 | Jun 28, 2012 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for MYRBETRIQ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Apgdi | MYRBETRIQ | mirabegron | TABLET, EXTENDED RELEASE;ORAL | 202611-001 | Jun 28, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Apgdi | MYRBETRIQ | mirabegron | TABLET, EXTENDED RELEASE;ORAL | 202611-002 | Jun 28, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Apgdi | MYRBETRIQ | mirabegron | TABLET, EXTENDED RELEASE;ORAL | 202611-001 | Jun 28, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Apgdi | MYRBETRIQ | mirabegron | TABLET, EXTENDED RELEASE;ORAL | 202611-002 | Jun 28, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for MYRBETRIQ
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Astellas Pharma Europe B.V. | Betmiga | mirabegron | EMEA/H/C/002388 Symptomatic treatment of urgency.Increased micturition frequency and / or urgency incontinence as may occur in adult patients with overactive-bladder syndrome. |
Authorised | no | no | no | 2012-12-20 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for MYRBETRIQ
When does loss-of-exclusivity occur for MYRBETRIQ?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Canada
Patent: 04298
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0121078
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 13670
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 16021
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 16021
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 2009057685
Estimated Expiration: ⤷ Try a Trial
Patent: 63170
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 16021
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 16021
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 16021
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 93525
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering MYRBETRIQ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2495666 | ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ ДЛЯ МОДИФИЦИРОВАННОГО ВЫСВОБОЖДЕНИЯ (MODIFIED RELEASE PHARMACEUTICAL COMPOSITION) | ⤷ Try a Trial |
| Hungary | 229295 | STABLE MEDICINAL COMPOSITIONS FOR ORAL USE | ⤷ Try a Trial |
| Norway | 316673 | ⤷ Try a Trial | |
| Poland | 369874 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for MYRBETRIQ
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1028111 | 300598 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: MIRABEGRON EN ZOUTEN ERVAN; REGISTRATION NO/DATE: EU/1/12/809/001-014 20121220 |
| 1559427 | 122013000047 | Germany | ⤷ Try a Trial | PRODUCT NAME: MIRABEGRON ODER EIN SALZ DAVON; NAT. REGISTRATION NO/DATE: EU /1/12/809/001-014 20121220; FIRST REGISTRATION: EU EU/1/12/809/001-014 20121220 |
| 1028111 | CA 2013 00029 | Denmark | ⤷ Try a Trial | PRODUCT NAME: MIRABEGRON OR SALTS THEREOF; REG. NO/DATE: EU/1/12/809/001-014 20121220 |
| 1559427 | 2013C/040 | Belgium | ⤷ Try a Trial | PRODUCT NAME: MIRABEGRON OU L'UN DES SES SELS; AUTHORISATION NUMBER AND DATE: EU/1/12/809/001 20130107 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


