LO LOESTRIN FE Drug Patent Profile
✉ Email this page to a colleague
When do Lo Loestrin Fe patents expire, and when can generic versions of Lo Loestrin Fe launch?
Lo Loestrin Fe is a drug marketed by Apil and is included in one NDA. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has nine patent family members in seven countries.
The generic ingredient in LO LOESTRIN FE is ethinyl estradiol; norethindrone acetate. There are twenty-six drug master file entries for this compound. Twenty-four suppliers are listed for this compound. Additional details are available on the ethinyl estradiol; norethindrone acetate profile page.
DrugPatentWatch® Generic Entry Outlook for Lo Loestrin Fe
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
There is one tentative approval for the generic drug (ethinyl estradiol; norethindrone acetate), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for LO LOESTRIN FE
| International Patents: | 9 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 5 |
| Clinical Trials: | 7 |
| Patent Applications: | 172 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for LO LOESTRIN FE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for LO LOESTRIN FE |
| What excipients (inactive ingredients) are in LO LOESTRIN FE? | LO LOESTRIN FE excipients list |
| DailyMed Link: | LO LOESTRIN FE at DailyMed |
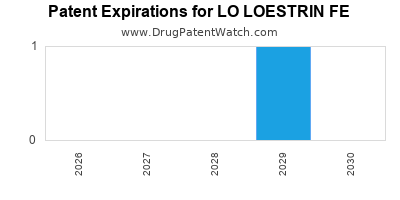
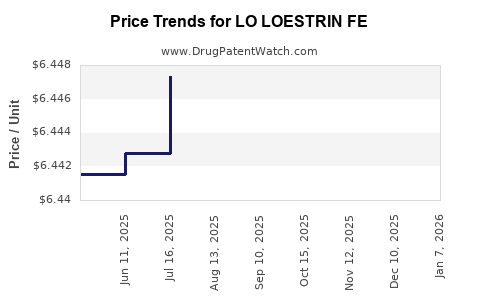
See drug prices for LO LOESTRIN FE
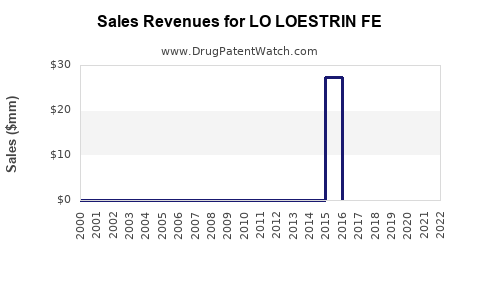
Recent Clinical Trials for LO LOESTRIN FE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Bristol-Myers Squibb | Phase 1 |
| Purdue Pharma LP | Phase 1 |
| Eisai Inc. | Phase 1 |
Anatomical Therapeutic Chemical (ATC) Classes for LO LOESTRIN FE
Paragraph IV (Patent) Challenges for LO LOESTRIN FE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| LO LOESTRIN FE | Tablets | ethinyl estradiol; norethindrone acetate | 1 mg/0.01 mg, 0.01 mg and 75 mg | 022501 | 1 | 2011-04-29 |
US Patents and Regulatory Information for LO LOESTRIN FE
LO LOESTRIN FE is protected by one US patents.
Patents protecting LO LOESTRIN FE
Extended estrogen dosing contraceptive regimen
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: LO LOESTRIN FE IS INDICATED FOR THE PREVENTION OF PREGNANCY IN WOMEN WHO ELECT TO USE ORAL CONTRACEPTIVES AS A METHOD OF CONTRACEPTION
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apil | LO LOESTRIN FE | ethinyl estradiol; norethindrone acetate | TABLET;ORAL | 022501-001 | Oct 21, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for LO LOESTRIN FE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Apil | LO LOESTRIN FE | ethinyl estradiol; norethindrone acetate | TABLET;ORAL | 022501-001 | Oct 21, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for LO LOESTRIN FE
When does loss-of-exclusivity occur for LO LOESTRIN FE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Canada
Patent: 05299
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1189015
Estimated Expiration: ⤷ Try a Trial
Patent: 4248639
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 77062
Estimated Expiration: ⤷ Try a Trial
Patent: 05266
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 05468
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 6656
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 07013137
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering LO LOESTRIN FE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Israel | 186656 | METHOD OF CONTRACEPTION AND A CONTRACEPTION KIT | ⤷ Try a Trial |
| Canada | 2605299 | REGIME CONTRACEPTIF DE DOSAGE D'ESTROGENES ETENDU (EXTENDED ESTROGEN DOSING CONTRACEPTIVE REGIMEN) | ⤷ Try a Trial |
| Hong Kong | 1205468 | 延長的雌激素定量給藥避孕療法 (EXTENDED ESTROGEN DOSING CONTRACEPTIVE REGIMEN) | ⤷ Try a Trial |
| Mexico | 2007013137 | REGIMEN ANTICONCEPTIVO CON DOSIFICACION DE ESTROGENO AMPLIADA. (EXTENDED ESTROGEN DOSING CONTRACEPTIVE REGIMEN.) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2006115871 | ⤷ Try a Trial | |
| European Patent Office | 2305266 | Régime contraceptif de dosage d strogènes étendu (Extended estrogen dosing contraceptive regimen) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for LO LOESTRIN FE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | CA 2016 00016 | Denmark | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL OG ETHINYLOESTRADIOL; NAT. REG. NO/DATE: 56336 20151105; FIRST REG. NO/DATE: SK 17/0017/15-S 20150211 |
| 0136011 | 2000C/027 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOLUM / NORETHISTERONI ACETAS; NAT. REGISTRATION NO/DATE: 19 IS 106 F3 20000911; FIRST REGISTRATION: NL RVG 23909 19991124 |
| 1453521 | 15C0050 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL ET MELANGE DE LEVONORGESTREL ET ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: NL 42237 20150320; FIRST REGISTRATION: SK - 17/0017/15-S 20150129 |
| 0771217 | 07C0001 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL BETADEX CLATHRATE; NAT. REGISTRATION NO/DATE: NL 32343 20060710; FIRST REGISTRATION: NL - RVG 31781 20050804 |
| 1453521 | 93156 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL ET ETHINYLESTRADIOL; FIRST REGISTRATION DATE: 20150211 |
| 1453521 | 300814 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL EN ETHINYLESTRADIOL; NATIONAL REGISTRATION NO/DATE: RVG 117453 20151211; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


