Progesterone - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for progesterone and what is the scope of patent protection?
Progesterone
is the generic ingredient in six branded drugs marketed by Amneal Pharms Ny, Bionpharma, Dr Reddys, Eugia Pharma, Sofgen Pharms, Teva Pharms, Xiromed, Virtus, Allergan, Accord Hlthcare, Actavis Labs Ut Inc, Am Regent, Fresenius Kabi Usa, Hikma Farmaceutica, Lilly, Alza, Ferring, and Ferring Pharms Inc, and is included in twenty NDAs. There are three patents protecting this compound. Additional information is available in the individual branded drug profile pages.Progesterone has fifty-three patent family members in twenty-five countries.
There are fifty-seven drug master file entries for progesterone. Twenty-five suppliers are listed for this compound.
Summary for progesterone
| International Patents: | 53 |
| US Patents: | 3 |
| Tradenames: | 6 |
| Applicants: | 18 |
| NDAs: | 20 |
| Drug Master File Entries: | 57 |
| Finished Product Suppliers / Packagers: | 25 |
| Raw Ingredient (Bulk) Api Vendors: | 116 |
| Clinical Trials: | 1,115 |
| Patent Applications: | 7,265 |
| Formulation / Manufacturing: | see details |
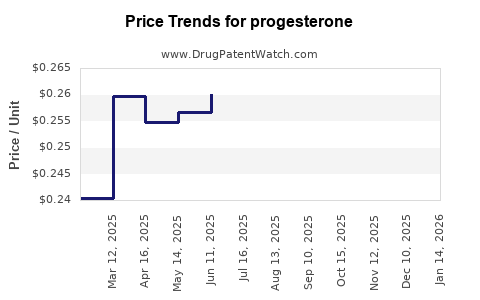
| Drug Prices: | Drug price trends for progesterone |
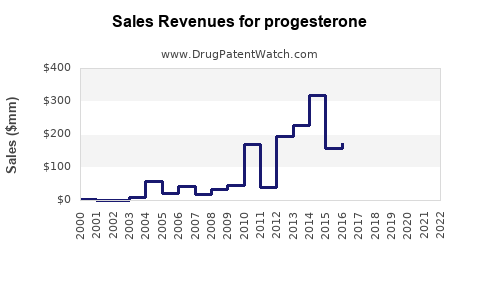
| Drug Sales Revenues: | Drug sales revenues for progesterone |
| What excipients (inactive ingredients) are in progesterone? | progesterone excipients list |
| DailyMed Link: | progesterone at DailyMed |
Recent Clinical Trials for progesterone
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Fayoum University | N/A |
| Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA) | Phase 4 |
| Fundación Santiago Dexeus Font | Phase 3 |
Pharmacology for progesterone
| Drug Class | Progesterone |
Medical Subject Heading (MeSH) Categories for progesterone
US Patents and Regulatory Information for progesterone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferring Pharms Inc | MILPROSA | progesterone | SYSTEM;VAGINAL | 201110-001 | Apr 29, 2020 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Lilly | PROGESTERONE | progesterone | INJECTABLE;INJECTION | 009238-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Xiromed | PROGESTERONE | progesterone | CAPSULE;ORAL | 205229-001 | Oct 20, 2017 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Eugia Pharma | PROGESTERONE | progesterone | CAPSULE;ORAL | 211285-001 | Oct 26, 2018 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Xiromed | PROGESTERONE | progesterone | CAPSULE;ORAL | 205229-002 | Oct 20, 2017 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Actavis Labs Ut Inc | PROGESTERONE | progesterone | INJECTABLE;INJECTION | 017362-002 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for progesterone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ferring | ENDOMETRIN | progesterone | INSERT;VAGINAL | 022057-001 | Jun 21, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Allergan | CRINONE | progesterone | GEL;VAGINAL | 020701-002 | Jul 31, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Allergan | CRINONE | progesterone | GEL;VAGINAL | 020701-002 | Jul 31, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Allergan | CRINONE | progesterone | GEL;VAGINAL | 020701-001 | Jul 31, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Ferring | ENDOMETRIN | progesterone | INSERT;VAGINAL | 022057-001 | Jun 21, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| Ferring | ENDOMETRIN | progesterone | INSERT;VAGINAL | 022057-001 | Jun 21, 2007 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for progesterone
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Singapore | 187529 | MONOLITHIC INTRAVAGINAL RINGS COMPRISING PROGESTERONE AND METHODS OF MAKING AND USES THEREOF | ⤷ Try a Trial |
| Israel | 221988 | טבעות מונוליטיות למתן נרתיקי המכילות פרוגסטרון, שיטות להכנתן ושימושים בהן (Monolithic intravaginal rings comprising progesterone and methods of making and using the same) | ⤷ Try a Trial |
| Spain | 2645630 | ⤷ Try a Trial | |
| China | 104146948 | Monolithic intravaginal rings comprising progesterone and methods of making and uses thereof | ⤷ Try a Trial |
| Canada | 2713943 | ANNEAUX INTRAVAGINAUX MONOLITHIQUES COMPRENANT DE LA PROGESTERONE ; PROCEDES DE FABRICATION ET UTILISATIONS (MONOLITHIC INTRAVAGINAL RINGS COMPRISING PROGESTERONE AND METHODS OF MAKING AND USES THEREOF) | ⤷ Try a Trial |
| Mexico | 337634 | ANILLOS INTRAVAGINALES MONOLITICOS QUE COMPRENDEN PROGESTERONA Y METODOS DE MANUFACTURA Y USO DE LOS MISMOS. (MONOLITHIC INTRAVAGINAL RINGS COMPRISING PROGESTERONE AND METHODS OF MAKING AND USES THEREOF.) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for progesterone
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2782584 | 301153 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTAINING BOTH ESTRADIOL (17SS-ESTRADIOL), OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE OR SOLVATE THEREOF (INCLUDING IN HEMIHYDRATE FORM), AND PROGESTERONE; NATIONAL REGISTRATION NO/DATE: RVG 125821 20210611; FIRST REGISTRATION: BE BE582231 20210406 |
| 2782584 | LUC00245 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTENANT A LA FOIS DE L'ESTRADIOL (17SS-ESTRADIOL), EVENTUELLEMENT SOUS FORME D'UN SEL, HYDRATE OU SOLVATE PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI (Y COMPRIS SOUS FORME HEMIHYDRATEE), ET DE LA PROGESTERONE; AUTHORISATION NUMBER AND DATE: BE582231 20210701 |
| 0113964 | 97C0037 | Belgium | ⤷ Try a Trial | PRODUCT NAME: OESTROGENES EQUINS CONJUGUES; ACETATE DE MEDROXYPROGESTERONE; NAT. REGISTRATION NO/DATE: NL 19569 19950301; FIRST REGISTRATION: CH - 52647 01 010 19940826 |
| 2782584 | 132021000000197 | Italy | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOLO (17SS-ESTRADIOLO) IN PARTICOLARE NELLA FORMA EMIIDRATA, E PROGESTERONE COMPRENDENTI LE VARIE FORME DI ESTRADIOLO (17SS-ESTRADIOLO), QUALI LE FORME IDRATE E SOLVATATE, INCLUDENDO LA FORMA EMIIDRATA, ED I SUOI SALI.(BIJUVA); AUTHORISATION NUMBER(S) AND DATE(S): BE582231, 20210406;048335018 -048335020, 20210517 |
| 2782584 | 21C1058 | France | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTENANT A LA FOIS DE L'ESTRADIOL (17SS-ESTRADIOL), Y COMPRIS SOUS FORME HEMIHYDRATEE, ET DE LA PROGESTERONE; NAT. REGISTRATION NO/DATE: NL51886 20210421; FIRST REGISTRATION: BE - BE582231 20210406 |
| 2782584 | 2021C/558 | Belgium | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTENANT A LA FOIS DE L'ESTRADIOL (17--ESTRADIOL), EVENTUELLEMENT SOUS FORME D'UN SEL, HYDRATE OU SOLVATE PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI (Y COMPRIS SOUS FORME HEMIHYDRATEE), ET DE LA PROGESTERONE; AUTHORISATION NUMBER AND DATE: BE582231 20210406 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.