Loteprednol etabonate - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for loteprednol etabonate and what is the scope of freedom to operate?
Loteprednol etabonate
is the generic ingredient in seven branded drugs marketed by Bausch And Lomb Inc, Sentiss, Slayback Pharma Llc, Sun Pharm, Bausch And Lomb, Alcon Labs Inc, Pharmos, and Lupin Ltd, and is included in sixteen NDAs. There are twenty-one patents protecting this compound. Additional information is available in the individual branded drug profile pages.Loteprednol etabonate has one hundred and eleven patent family members in sixteen countries.
There are ten drug master file entries for loteprednol etabonate. Seven suppliers are listed for this compound.
Summary for loteprednol etabonate
| International Patents: | 111 |
| US Patents: | 21 |
| Tradenames: | 7 |
| Applicants: | 8 |
| NDAs: | 16 |
| Drug Master File Entries: | 10 |
| Finished Product Suppliers / Packagers: | 7 |
| Raw Ingredient (Bulk) Api Vendors: | 63 |
| Clinical Trials: | 47 |
| Patent Applications: | 6,067 |
| Formulation / Manufacturing: | see details |
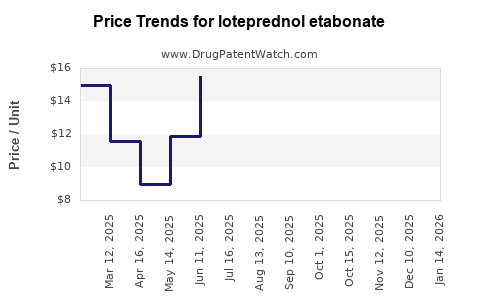
| Drug Prices: | Drug price trends for loteprednol etabonate |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for loteprednol etabonate |
| What excipients (inactive ingredients) are in loteprednol etabonate? | loteprednol etabonate excipients list |
| DailyMed Link: | loteprednol etabonate at DailyMed |
Recent Clinical Trials for loteprednol etabonate
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Kala Pharmaceuticals, Inc. | Phase 4 |
| Price Vision Group | Phase 4 |
| Thomas Chester, OD | Phase 4 |
Pharmacology for loteprednol etabonate
| Drug Class | Corticosteroid |
| Mechanism of Action | Corticosteroid Hormone Receptor Agonists |
Medical Subject Heading (MeSH) Categories for loteprednol etabonate
Paragraph IV (Patent) Challenges for LOTEPREDNOL ETABONATE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| LOTEMAX SM | Ophthalmic Gel | loteprednol etabonate | 0.38% | 208219 | 1 | 2022-11-14 |
US Patents and Regulatory Information for loteprednol etabonate
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcon Labs Inc | INVELTYS | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 210565-001 | Aug 22, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Alcon Labs Inc | INVELTYS | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 210565-001 | Aug 22, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Alcon Labs Inc | EYSUVIS | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 210933-001 | Oct 26, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bausch And Lomb | LOTEMAX | loteprednol etabonate | OINTMENT;OPHTHALMIC | 200738-001 | Apr 15, 2011 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sentiss | LOTEPREDNOL ETABONATE | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 207609-001 | Apr 17, 2019 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Alcon Labs Inc | EYSUVIS | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 210933-001 | Oct 26, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for loteprednol etabonate
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pharmos | LOTEMAX | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 020841-001 | Mar 9, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bausch And Lomb | LOTEMAX | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 020583-001 | Mar 9, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bausch And Lomb | ALREX | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 020803-001 | Mar 9, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bausch And Lomb | ALREX | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 020803-001 | Mar 9, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bausch And Lomb Inc | LOTEMAX | loteprednol etabonate | GEL;OPHTHALMIC | 202872-001 | Sep 28, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Pharmos | LOTEMAX | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 020841-001 | Mar 9, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for loteprednol etabonate
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20170105610 | 안과용 현탁액 조성물 | ⤷ Try a Trial |
| New Zealand | 728721 | Pharmaceutical nanoparticles showing improved mucosal transport | ⤷ Try a Trial |
| Japan | 2015519331 | 改善された粘膜輸送を示す医薬用ナノ粒子 | ⤷ Try a Trial |
| Chile | 2019003432 | Composición farmacéutica oftálmica que comprende nanopartículas mucopenetrantes, cuyo núcleo comprende un agente activo farmacéutico seleccionado entre un inhibidor de ciclooxigenasa (cox) o un fármaco antiinflamatorio no esterioidal (aine), y está recubierto con un polímero modificador de superficie; y uso para tratar un trastorno del ojo (divisional de solicitud cl 2956-2014). | ⤷ Try a Trial |
| South Korea | 20150004909 | PHARMACEUTICAL NANOPARTICLES SHOWING IMPROVED MUCOSAL TRANSPORT | ⤷ Try a Trial |
| Canada | 2975106 | COMPOSITION DE SUSPENSION OPHTALMIQUE (OPHTHALMIC SUSPENSION COMPOSITION) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.