Eszopiclone - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for eszopiclone and what is the scope of patent protection?
Eszopiclone
is the generic ingredient in two branded drugs marketed by Aurobindo Pharma, Chartwell Rx, Dr Reddys, Glenmark Generics, Hetero Labs Ltd V, Hikma, Ipca Labs Ltd, Lupin Ltd, Macleods Pharms Ltd, Mylan Pharms Inc, Nostrum Labs Inc, Orbion Pharms, Sun Pharm, Teva, and Woodward, and is included in fifteen NDAs. Additional information is available in the individual branded drug profile pages.There are twenty drug master file entries for eszopiclone. Thirty-two suppliers are listed for this compound.
Summary for eszopiclone
| US Patents: | 0 |
| Tradenames: | 2 |
| Applicants: | 15 |
| NDAs: | 15 |
| Drug Master File Entries: | 20 |
| Finished Product Suppliers / Packagers: | 32 |
| Raw Ingredient (Bulk) Api Vendors: | 60 |
| Clinical Trials: | 68 |
| Patent Applications: | 6,810 |
| Formulation / Manufacturing: | see details |
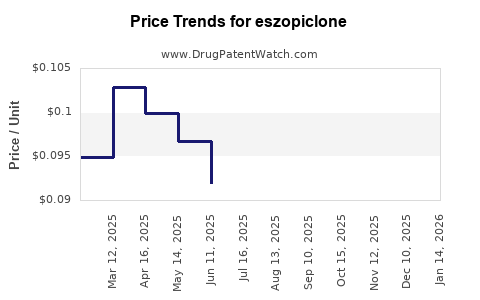
| Drug Prices: | Drug price trends for eszopiclone |
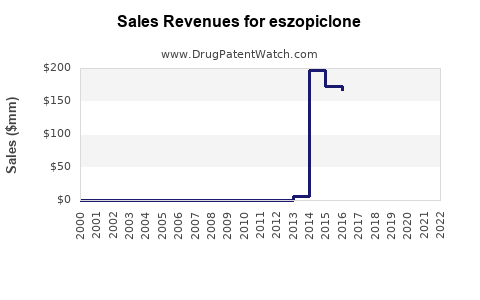
| Drug Sales Revenues: | Drug sales revenues for eszopiclone |
| What excipients (inactive ingredients) are in eszopiclone? | eszopiclone excipients list |
| DailyMed Link: | eszopiclone at DailyMed |
Recent Clinical Trials for eszopiclone
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Brigham and Women's Hospital | Phase 1/Phase 2 |
| Laboratorios Andromaco S.A. | Phase 1 |
| VA Office of Research and Development | Phase 3 |
Medical Subject Heading (MeSH) Categories for eszopiclone
Paragraph IV (Patent) Challenges for ESZOPICLONE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| LUNESTA | Tablets | eszopiclone | 1 mg, 2 mg and 3 mg | 021476 | 10 | 2008-12-15 |
US Patents and Regulatory Information for eszopiclone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mylan Pharms Inc | ESZOPICLONE | eszopiclone | TABLET;ORAL | 091151-001 | Mar 26, 2013 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Mylan Pharms Inc | ESZOPICLONE | eszopiclone | TABLET;ORAL | 091151-003 | Mar 26, 2013 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Chartwell Rx | ESZOPICLONE | eszopiclone | TABLET;ORAL | 091165-001 | Jul 14, 2011 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Dr Reddys | ESZOPICLONE | eszopiclone | TABLET;ORAL | 091024-003 | Apr 15, 2014 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for eszopiclone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Woodward | LUNESTA | eszopiclone | TABLET;ORAL | 021476-001 | Dec 15, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| Woodward | LUNESTA | eszopiclone | TABLET;ORAL | 021476-001 | Dec 15, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| Woodward | LUNESTA | eszopiclone | TABLET;ORAL | 021476-002 | Dec 15, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| Woodward | LUNESTA | eszopiclone | TABLET;ORAL | 021476-001 | Dec 15, 2004 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |