Drospirenone - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for drospirenone and what is the scope of patent protection?
Drospirenone
is the generic ingredient in twenty branded drugs marketed by Exeltis Usa Inc, Mayne Pharma, Bayer Hlthcare, Barr, Glenmark Pharms Ltd, Hetero Labs, Hlthcare, Jubilant Cadista, Mylan Labs Ltd, Watson Labs, Sun Pharm, Aurobindo Pharma Ltd, Xiromed, Novast Labs, Lupin Ltd, Apotex, Dr Reddys Labs Sa, Naari Pte Ltd, and Watson Labs Inc, and is included in thirty-seven NDAs. There are eighteen patents protecting this compound. Additional information is available in the individual branded drug profile pages.Drospirenone has sixty-five patent family members in twenty-nine countries.
There are eleven drug master file entries for drospirenone. One supplier is listed for this compound. There is one tentative approval for this compound.
Summary for drospirenone
| International Patents: | 65 |
| US Patents: | 18 |
| Tradenames: | 20 |
| Applicants: | 19 |
| NDAs: | 37 |
| Drug Master File Entries: | 11 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 79 |
| Clinical Trials: | 98 |
| Patent Applications: | 3,354 |
| Formulation / Manufacturing: | see details |
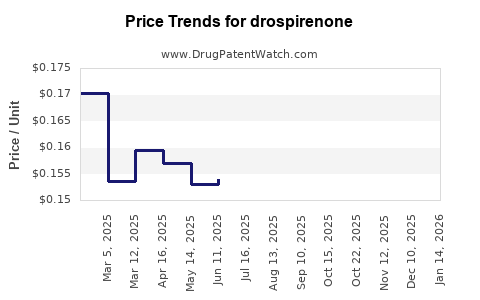
| Drug Prices: | Drug price trends for drospirenone |
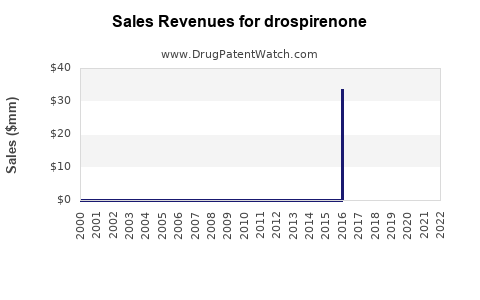
| Drug Sales Revenues: | Drug sales revenues for drospirenone |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for drospirenone |
| What excipients (inactive ingredients) are in drospirenone? | drospirenone excipients list |
| DailyMed Link: | drospirenone at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for drospirenone
Generic Entry Date for drospirenone*:
Constraining patent/regulatory exclusivity:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for drospirenone
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Colorado, Denver | Phase 2 |
| Bristol Myers Squibb Company (BMS) | Phase 1 |
| Janssen Pharmaceutica N.V., Belgium | Phase 1 |
Generic filers with tentative approvals for DROSPIRENONE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 4MG | TABLET;ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Paragraph IV (Patent) Challenges for DROSPIRENONE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SLYND | Tablets | drospirenone | 4 mg | 211367 | 1 | 2022-01-07 |
US Patents and Regulatory Information for drospirenone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jubilant Cadista | DROSPIRENONE AND ETHINYL ESTRADIOL | drospirenone; ethinyl estradiol | TABLET;ORAL-28 | 210017-001 | Sep 10, 2018 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bayer Hlthcare | ANGELIQ | drospirenone; estradiol | TABLET;ORAL | 021355-001 | Feb 29, 2012 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Exeltis Usa Inc | SLYND | drospirenone | TABLET;ORAL | 211367-001 | May 23, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for drospirenone
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20170085604 | 드로스피레논을 포함하는 약학적 조성물 및 피임용 키트 (Pharmaceutical composition comprising drospirenone and contraceptive kit) | ⤷ Try a Trial |
| Spain | 2777886 | ⤷ Try a Trial | |
| European Patent Office | 2588114 | COMPOSITION PHARMACEUTIQUE CONTENANT DE LA DROSPIRÉNONE ET KIT CONTRACEPTIF (PHARMACEUTICAL COMPOSITION COMPRISING DROSPIRENONE AND CONTRACEPTIVE KIT) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for drospirenone
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3632448 | PA2022513 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONAS; REGISTRATION NO/DATE: LT/1/21/4721/001-004 20210419 |
| 2588114 | 19/2020 | Austria | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON; NAT. REGISTRATION NO/DATE: 139227 20191206; FIRST REGISTRATION: DK 31332 (MITTEILUNG) 20191022 |
| 3632448 | 301186 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SLINDA; NATIONAL REGISTRATION NO/DATE: RVG 127386 20210317; FIRST REGISTRATION: DK 61678 20191022 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |