Ciprofloxacin - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for ciprofloxacin and what is the scope of patent protection?
Ciprofloxacin
is the generic ingredient in seventeen branded drugs marketed by Bayer Hlthcare, Chartwell, Alk Abello, Bayer Pharms, Baxter Hlthcare Corp, Bedford Labs, Dr Reddys, Fresenius Kabi Usa, Hikma Farmaceutica, Hospira, Baxter Hlthcare, Bedford, Inforlife, Teva Pharms, Sandoz, Akorn, Altaire Pharms Inc, Amring Pharms, Fdc Ltd, Pai Holdings Pharm, Rising, Rubicon, Watson Labs Inc, Wraser Pharms, Depomed Inc, Aiping Pharm Inc, Amneal, Aurobindo Pharma, Barr, Carlsbad, Dr Reddys Labs Ltd, Hikma, Ivax Sub Teva Pharms, Mylan, Nostrum Labs, Pliva, Sun Pharm Inds Ltd, Taro, Teva, Unique, Watson Labs, Yiling, Laboratorios Salvat, Anchen Pharms, Ani Pharms, Fosun Pharma, Sentiss, and Sun Pharm, and is included in sixty-five NDAs. There are ten patents protecting this compound and one Paragraph IV challenge. Additional information is available in the individual branded drug profile pages.Ciprofloxacin has one hundred and fifty patent family members in twenty countries.
There are thirty-four drug master file entries for ciprofloxacin. Five suppliers are listed for this compound. There are four tentative approvals for this compound.
Summary for ciprofloxacin
| International Patents: | 150 |
| US Patents: | 10 |
| Tradenames: | 17 |
| Applicants: | 48 |
| NDAs: | 65 |
| Drug Master File Entries: | 34 |
| Finished Product Suppliers / Packagers: | 5 |
| Raw Ingredient (Bulk) Api Vendors: | 123 |
| Clinical Trials: | 297 |
| Patent Applications: | 7,194 |
| Formulation / Manufacturing: | see details |
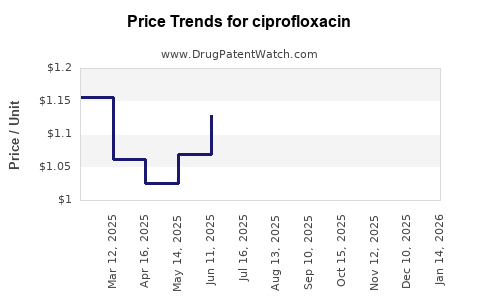
| Drug Prices: | Drug price trends for ciprofloxacin |
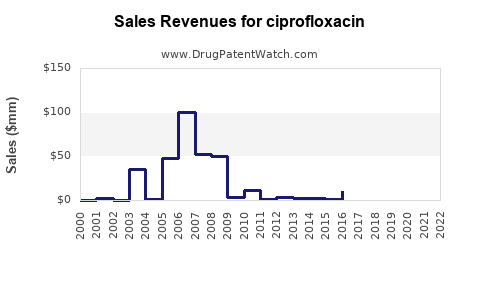
| Drug Sales Revenues: | Drug sales revenues for ciprofloxacin |
| What excipients (inactive ingredients) are in ciprofloxacin? | ciprofloxacin excipients list |
| DailyMed Link: | ciprofloxacin at DailyMed |
Recent Clinical Trials for ciprofloxacin
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Missouri-Columbia | Phase 4 |
| Sanofi | Phase 1 |
| Servier Bio-Innovation LLC | Phase 1 |
Generic filers with tentative approvals for CIPROFLOXACIN
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | EQ 750MG BASE | TABLET;ORAL |
| ⤷ Try a Trial | ⤷ Try a Trial | EQ 500MG BASE | TABLET;ORAL |
| ⤷ Try a Trial | ⤷ Try a Trial | EQ 250MG BASE | TABLET;ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for ciprofloxacin
| Drug Class | Fluoroquinolone Antibacterial Quinolone Antimicrobial |
| Mechanism of Action | Cytochrome P450 1A2 Inhibitors |
Medical Subject Heading (MeSH) Categories for ciprofloxacin
Paragraph IV (Patent) Challenges for CIPROFLOXACIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| CIPRO | Oral Suspension | ciprofloxacin | 250 mg/5 mL and 500 mg/ 5 mL | 020780 | 1 | 2009-10-16 |
US Patents and Regulatory Information for ciprofloxacin
Expired US Patents for ciprofloxacin
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | CIPRO | ciprofloxacin | FOR SUSPENSION;ORAL | 020780-001 | Sep 26, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bayer Hlthcare | CIPRO | ciprofloxacin | FOR SUSPENSION;ORAL | 020780-002 | Sep 26, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bayer Hlthcare | CIPRO | ciprofloxacin | FOR SUSPENSION;ORAL | 020780-002 | Sep 26, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bayer Hlthcare | CIPRO | ciprofloxacin | FOR SUSPENSION;ORAL | 020780-001 | Sep 26, 1997 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bayer Hlthcare | CIPRO IN DEXTROSE 5% IN PLASTIC CONTAINER | ciprofloxacin | INJECTABLE;INJECTION | 019857-001 | Dec 26, 1990 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bayer Hlthcare | CIPRO IN DEXTROSE 5% IN PLASTIC CONTAINER | ciprofloxacin | INJECTABLE;INJECTION | 019857-001 | Dec 26, 1990 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ciprofloxacin
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2017119723 | 耳の疾患と疾病を処置するための耳の製剤と関連する応用 (AURIS FORMULATION FOR TREATING OTIC DISEASE AND CONDITION AND APPLICATION RELATED THERETO) | ⤷ Try a Trial |
| United Kingdom | 2461962 | NMDA receptor antagonists for the treatment of ear disorders | ⤷ Try a Trial |
| South Korea | 101390607 | ⤷ Try a Trial | |
| South Korea | 102340754 | ⤷ Try a Trial | |
| Russian Federation | 2493828 | МОДУЛИРУЮЩИЕ АПОПТОЗ КОМПОЗИЦИИ С КОНТРОЛИРУЕМЫМ ВЫСВОБОЖДЕНИЕМ И СПОСОБЫ ЛЕЧЕНИЯ ЗАБОЛЕВАНИЙ УХА (APOPTOSIS MODULATING COMPOSITIONS WITH CONTROLLED RELEASE AND METHODS OF TREATING EAR DISEASES) | ⤷ Try a Trial |
| Canada | 2721927 | PREPARATIONS AURICULAIRES DE TRAITEMENT DE MALADIES ET ETATS OTIQUES (AURIS FORMULATIONS FOR TREATING OTIC DISEASES AND CONDITIONS) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ciprofloxacin
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1429780 | SPC/GB12/058 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF CIPROFLOXACIN AND DEXAMETHASONE, PREFERABLY CIPROFLOXACIN HYDROCHLORIDE AND DEXAMETHASONE; REGISTERED: DK DE/11/3337/001/DC 20120808; UK PL000649/0381-0001 20121003 |
| 1429780 | 132013902137451 | Italy | ⤷ Try a Trial | PRODUCT NAME: CIPROFLOXACINA E DESAMETASONE(CILODEX); AUTHORISATION NUMBER(S) AND DATE(S): 48976, 20120810;041182015/M, 20121106 |
| 1429780 | 13C0012 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE CIPROFLOXACINE ET DE DEXAMETHASONE, EN PARTICULIER DE CHLORHYDRATE DE CIPROFLOXACINE ET DE DEXAMETHASONE; NAT. REGISTRATION NO/DATE: NL 41308 20121214; FIRST REGISTRATION: 48976 20120808 |
| 1429780 | 122012000070 | Germany | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION AUS CIPROFLOXACIN UND DEXAMETHASON, INSBESONDERE CIPROFLOXACINHYDROCHLORID UND DEXAMETHASON; NAT. REGISTRATION NO/DATE: 85150.00. 00 20120830; FIRST REGISTRATION: DAENEMARK 48976 20120808 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.