Atorvastatin calcium - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for atorvastatin calcium and what is the scope of patent protection?
Atorvastatin calcium
is the generic ingredient in five branded drugs marketed by Cmp Dev Llc, Accord Hlthcare, ACI, Agnitio, Alkem Labs Ltd, Anbison Lab, Apotex Inc, Aurobindo Pharma Ltd, Biocon Pharma, Cadila Pharms Ltd, Dr Reddys, Dr Reddys Labs Ltd, Graviti Pharms, Hetero Labs Ltd V, Invagen Pharms, Lannett Co Inc, Laurus, Lepu Pharm, Lupin Ltd, Mankind Pharma, Micro Labs Ltd India, MSN, Mylan Pharms Inc, Perrigo R And D, Sandoz Inc, Sciegen Pharms Inc, Sun Pharm Inds Ltd, Teva Pharms, Teva Pharms Usa, Umedica, Zydus Pharms, Upjohn, Organon, and Althera Pharms, and is included in thirty-five NDAs. There are two patents protecting this compound. Additional information is available in the individual branded drug profile pages.Atorvastatin calcium has two patent family members in two countries.
There are fifty-five drug master file entries for atorvastatin calcium. Sixty-three suppliers are listed for this compound.
Summary for atorvastatin calcium
| International Patents: | 2 |
| US Patents: | 2 |
| Tradenames: | 5 |
| Applicants: | 34 |
| NDAs: | 35 |
| Drug Master File Entries: | 55 |
| Finished Product Suppliers / Packagers: | 63 |
| Raw Ingredient (Bulk) Api Vendors: | 85 |
| Clinical Trials: | 69 |
| Patent Applications: | 7,598 |
| Formulation / Manufacturing: | see details |
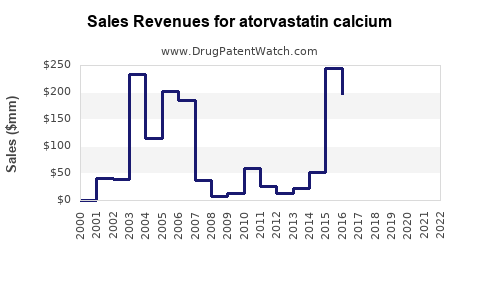
| Drug Sales Revenues: | Drug sales revenues for atorvastatin calcium |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for atorvastatin calcium |
| What excipients (inactive ingredients) are in atorvastatin calcium? | atorvastatin calcium excipients list |
| DailyMed Link: | atorvastatin calcium at DailyMed |
Recent Clinical Trials for atorvastatin calcium
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of California, San Francisco | Phase 2 |
| United States Department of Defense | Phase 2 |
| Ayub Teaching Hospital | N/A |
Pharmacology for atorvastatin calcium
| Drug Class | HMG-CoA Reductase Inhibitor |
| Mechanism of Action | Hydroxymethylglutaryl-CoA Reductase Inhibitors |
Medical Subject Heading (MeSH) Categories for atorvastatin calcium
US Patents and Regulatory Information for atorvastatin calcium
Expired US Patents for atorvastatin calcium
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-004 | Apr 7, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-004 | Apr 7, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-002 | Dec 17, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-001 | Dec 17, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-001 | Dec 17, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-004 | Apr 7, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-003 | Dec 17, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for atorvastatin calcium
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| World Intellectual Property Organization (WIPO) | 2017212409 | ⤷ Try a Trial | |
| European Patent Office | 3468606 | NOUVELLE COMPOSITION PHARMACEUTIQUE DE COMPOSÉ HYPOLIPIDÉMIANT (A NOVEL PHARMACEUTICAL COMPOSITION OF A LIPID LOWERING COMPOUND) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for atorvastatin calcium
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1003503 | C01003503/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: AMLODIPIN + ATORVASTATIN; REGISTRATION NUMBER/DATE: SWISSMEDIC 57633 05.07.2006 |
| 0247633 | 97C0118 | France | ⤷ Try a Trial | PRODUCT NAME: ATORVASTATINE CALCIQUE TRIHYDRATE; REGISTRATION NO/DATE IN FRANCE: NL 21960 DU 19970321; REGISTRATION NO/DATE AT EEC: PL 00018/0240 DU 19961107 |
| 0247633 | 62/1997 | Austria | ⤷ Try a Trial | PRODUCT NAME: ATORVASTATIN CALCIUM; NAT. REGISTRATION NO/DATE: 1-21926, 1-21927, 1-21928 19970411; FIRST REGISTRATION: GB PL 00018/0240 - PL 00018/0242 19961107 |
| 0720599 | 122014000088 | Germany | ⤷ Try a Trial | PRODUCT NAME: EZETIMIB ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON IN KOMBINATION MIT ATORVASTATIN ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON, INSBESONDERE ATORVASTATIN ALS ATORVASTATINCALCIUMTRIHYDRAT; NAT. REGISTRATION NO/DATE: 90223.00.00 20141113; FIRST REGISTRATION: FRANKREICH CIS: 6 928 917 6 20140912 |
| 0720599 | 300689 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: EZETIMIBE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, EN ATORVASTATINE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER ATORVASTATINE CALCIUM TRIHYDRATE; NATIONAL REGISTRATION NO/DATE: RVG114373-114376 20141027; FIRST REGISTRATION: FR 2014091200122 20140912 |
| 0720599 | 92545 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: EZETIMIBE EN COMBINAISON AVEC ATORVASTATINE OU LEURS SELS PHARMACEUTIQUEMENT ACCEPTEES, Y COMPRIS ATORVASTATINE SOUS FORME D'ATORVASTATINE CALCIQUE TRIHYDRATEE; FIRST REGISTRATION: 20140910 |
| 0720599 | CR 2014 00050 | Denmark | ⤷ Try a Trial | PRODUCT NAME: EZETIMIBE AND ATORVASTATIN OR PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, INCLUDING ATORVASTATIN AS ATORVASTATIN CALCIUM TRIHYDRATE; REG. NO/DATE: DE/H/3895-3898/001-004/DC 20140910 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.